Common Ions to Memorize 2014
advertisement

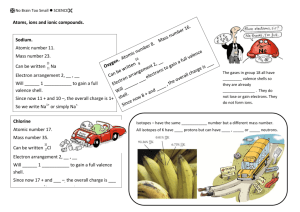
Honors Chemistry Common Ions to Memorize for Ion Mastery Quizzes Ammonium Acetate Hydroxide NH4+1 C2H3O2-1 OH-1 Phosphate Phosphite PO4-3 PO3-3 Sulfate Sulfite Sulfide SO4-2 SO3-2 S-2 Bicarbonate Carbonate HCO3-1 CO3-2 Perchlorate Chlorate Chlorite Hypochlorite Chloride ClO4-1 ClO3-1 ClO2-1 ClO-1 Cl-1 Nitrate Nitrite Nitride NO3-1 NO2-1 N-3 Silver Ag+1 Iodate Iodite Iodide IO3-1 IO2-1 I-1 Zinc Zn+2 Group 1 All +1 Oxide Fluoride Bromide O-2 F-1 Br-1 Group 2 All +2 Hints and Patterns: 1) Ions that end in “ide” or keep their name have one atom in the ion and match their charge on the P.T. a. Exception is Hydroxide 2) Ions that end in “ate” usually have 3 oxygens behind the root element and a charge of -1 a. Exceptions are Sulfate and Phosphate 3) Ions that end in “ite” have one less oxygen than the “ate” 4) Ions that start with “hypo” have one less oxygen than the “ite” 5) Ions that start with “per” have one more oxygen that the “ate” 6) Ions that start with “bi” have a hydrogen, one less oxygen, and a more positive charge 7) Ions with root elements have the same charge as their charge on the P.T. a. Exception is Nitrate/Nitride