A.P. Chemistry Rates of Reaction Chapter 12 : page 526 I. Chemical
advertisement

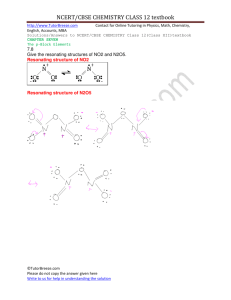
A.P. Chemistry Rates of Reaction I. Chemical Kinetics A. Definition- B. Requirements for a chemical reaction 1. 2. 3. II. Reaction Rates A. Rate of a reaction- B. Example: C. Example 1. Example 2 1 2 N2O5 (g) → 4 NO2 (g) + O2 (g) Chapter 12 : page 526 III. Factors which effect the rate of reactions A. Concentration of the reactants 1. a. b. 2. Effect of concentration on the reaction rate a. b. Example: If we double [NO2-] **3. We can express the overall concentration dependence using RATE LAWS a. Differential rate law or just Rate Law - determined experimentally b. Integrated rate law – discussed in Part V. IV. The Differential Rate Law A. The form of the rate law - for a general reaction: aA + bB cC + dD B. Determining the orders of a Rate Law Rate = k [A] Rate = k [A] C. k – 2 __________ order 2 __________ order Rate = k [A][B] __________ order Rate = k[A][B]2 __________ order Example 1: The initial rate of a hypothetical reaction: A + B → C was measured for several different starting concentrations of A and B, with the results given below: Exp No. 1 2 3 [A] 0.100 0.100 0.200 [B] 0.100 0.200 0.100 Initial rate M/sec 4.0 x 10-5 4.0 x 10-5 16.0 x 10-5 Using these data, determine (a) the rate law for the reaction, (b) the rate constant (c) the rate of the reaction when [A] = 0.050 M and [B] = 0.100 M. Example 2: Table 12.4 in textbook. 3 Example 3: Consider the reaction of peroxydisulfate ion with the iodide ion: S2O82- + 3 I- 2 SO42- + I3At a certain temperature the rate of disappearance of S2O82- varies with reactant concentration in the following manner: Experiment [S2O82-] [I-] Rate (mol/L•s) 1 0.018 0.036 2.6 x 10-6 2 0.027 0.036 3.9 x 10-6 3 0.036 0.056 7.8 x 10-6 4 0.050 0.072 1.4 x 10-5 a. Determine the rate law. b. Determine the value of k. c. How is the rate of disappearance of [S2O8]2- related to the rate of disappearance of [I-]? d. What is the rate of disappearance of I- when [S2O8]2- = 0.015 M and [I-] = 0.040 M? 4 V. The Integrated Rate Law A. First-Order Rate Law – 1. For a chemical reaction - 2. The equation: 3. The graph: 4. Half-life B. Second-Order Rate Law – 1. For a chemical reaction – 2. The equation: 3. The graph 4. Half-Life 5 Example Problems: 1. A first order rate constant for hydrolysis of a certain insecticide in water at 12oC is 1.45/yr. A quantity of this insecticide is washed into a lake in June, leading to an overall concentration of 5.0 x 10-7 g/cm3. Assuming the effective temperature of the lake is 12oC find a. The concentration of the insecticide in June of the following year b. How long it will take for the concentration of the insecticide to drop to 3.0 x 10-7 g/cm3 2. The decomposition of N2O5 in the gas phase was studied at constant temperature. 2 N2O5 (g) 4NO2 (g) + O2 (g) The following results were collected: 6 [N2O5] Time (s) 0.1000 0 0.0707 50 0.0500 100 0.0250 200 0.0125 300 0.00625 400 a. determine the order of this reaction b. calculate the value of the rate constant How to determine the Order using the graphing calculator: 1. Find the “catalog” button on your calculator and enter. Scroll down to the D’s and turn the diagnostic on. Press enter. 2. Plot the graphs of each order and determine which line is the straightest. a. STAT EDIT and enter the time into L1 and the concentration into L2 b. Go to L3 (use your arrow key to move up and highlight L3) and type in ln(L2) c. Go to L4 (use your arrow key to move up and highlight L4) and type in 1/L2 d. Press QUIT e. To see the graph be sure your STAT PLOT is ON and you have defined your X and Y. Press ZOOM and enter ZOOM STAT (at the bottom of the list). f. STAT CALC then LinReg(ax+b) (4) ENTER g. Type in L1 , L3 to see the stats for the first order plot h. Type in L1 , L4 to see the stats for the second order plot i. The line with the “r” (correlation) closer to 1 or -1 is the straighter line. j. “a” will be the slope and the value of the rate constant. Example 3: Consider this reaction: 2 C2H4 (g) C8H12 (g) [C4H6] Time (s) 0.01000 0 0.00625 1000 0.00476 1800 0.00370 2800 0.00313 3600 0.00270 4400 0.00241 5200 0.00208 6200 a. Is this reaction first order or second order? b. What is the value of the rate constant for the reaction? c. What is the half-life for the reaction under the conditions of this experiment? Example 4: Using Half-Life Equations with the Integrated Rate Law. A first order reaction is 38.5% complete in 480 seconds a. Calculate the rate constant b. Calculate the value of the half life c. How long will it take for the reaction to go to 25%? d. How long will it take for the reaction to go to 75%? e. How long will it take for the reaction to go to 95%? 7 VI. Defining the Molecularity of a Reaction A. Reaction Mechanism – most chemical reactions occur by a series of steps called the reaction mechanism. 1. Definition of reaction mechanism: The set of ____________ _____________, and their rates, that describe the _____________ by which a reaction occurs; the overall effect of the ____________ ____________ of the mechanism is given by the _________________ _______________ ____________________. 2. Elementary Steps- 3. For a reaction, the mechanism must meet two criteria for it to be a plausible mechanism: a. b. 4. The 3 kinds of steps: the number of particles that come together in each elementary step is know as the molecularity. a. b. c. 5. Some steps are fast and one step will be slow, the slow step is the _____________ ___________________ ________________. 6. Example 1: For the reaction- 2 NO2 + F2 2 NO2F A suggested mechanism for the above reaction is: Step 1: NO2 + F2 NO2F + FStep 2: NO2 + F- NO2F (slow) (fast) If this is the correct mechanism, what is the rate law? 8 7. Example 2: The rate law for the reaction: 2 ClO2 + F2 2 ClO2F is Rate = k[ClO2][F] Which of the following mechanisms is consistent with the rate law? Justify your answer. I. ClO2 + F2 ClO2F2 (fast) ClO2F2 ClO2F + F- (slow) ClO2 + F- ClO2F (fast) 8. Example 3: For the reaction: 2A+B C II. F2 2 F- (slow) 2(ClO2 + F- ClO2F) (fast) the following reaction data was collected: Experiment Rate of the formation of C M/s Initial [A] Initial [B] I II III IV 4.0 x 10-2 2.0 x 10-2 6.0 x 10-2 1.0 x 10-2 0.30 0.50 0.50 0.40 0.10 0.05 0.15 0.025 a. What is the rate law consistent with the above data? b. Calculate k and include the units. c. How long will it take the concentration of C to equal 0.10 M if the initial concentration of [A] = 0.4M and [B] = 0.3 M? d. Out of the mechanisms listed below, which is the most consistent with the observed kinetics? Present some kind of logical argument to accompany your choice. 1. A + B g M (slow) A + M g C (fast) 9 2. A + B M (fast) B + M C (slow) 3. B M (slow) A + M N (fast) N + B C (fast) VII. Temperature and the effect on the rate A. In general: when increasing the temperature by 10o the number of colliding particles increase so the rate increases. A 10o rise in temperature will double the reaction rate. B. Exothermic Reaction- for a reaction : A2 + B2 2 AB + energy (∆H is negative) EA- is the Activation Energy- the minimum energy that must be supplied by the reactants in a chemical reaction to overcome the barrier to the products. Activated Complex-Particular arrangement of reactants and products molecules at the point of maximum energy in the rate-determining step of a reaction. C. Endothermic Reaction: for a reaction : A2 + B2 + energy 2 AB (∆H is positive) VIII. Effect of a catalyst A. DefinitionB. Four Properties 1. Works by 2. Does not appear in the 3. Can be included in the 4. It is C. Effect on the mechanism: 10 D. How you can see a catalyst in a reaction: 1. Consider the following mechanism and determine the overall reaction. Step 1: Step 2: NO (g) + O3 NO2 + O2 O (g) + NO2 NO + O2 2. Example 2: Step 1: Step 2: H2O2 + I- H2O + IOH2O2 + IO- H2O + I- A(g) + B(g) → C(g) + D(g) For the gas-phase reaction represented above, the following experimental data were obtained. Experiment 1 2 3 4 Initial [A] (mol L−1) 0.033 0.034 0.136 0.202 Initial [B] (mol L−1) 0.034 0.137 0.136 0.233 Initial Reaction Rate (mol L−1 s−1) 6.67 × 10−4 1.08 × 10−2 1.07 × 10−2 ? (a) Determine the order of the reaction with respect to reactant A. Justify your answer. (b) Determine the order of the reaction with respect to reactant B. Justify your answer. (c) Write the rate law for the overall reaction. (d) Determine the value of the rate constant, k , for the reaction. Include units with your answer. (e) Calculate the initial reaction rate for experiment 4. (f) The following mechanism has been proposed for the reaction. Step 1: B + B → E + D slow Step 2: E + A B + C fast equilibrium Provide two reasons why the mechanism is acceptable. (g) In the mechanism in part (f), is species E a catalyst, or is it an intermediate? Justify your answer. 11