AP Chemistry Fall Final Exam Practice Worksheet
advertisement
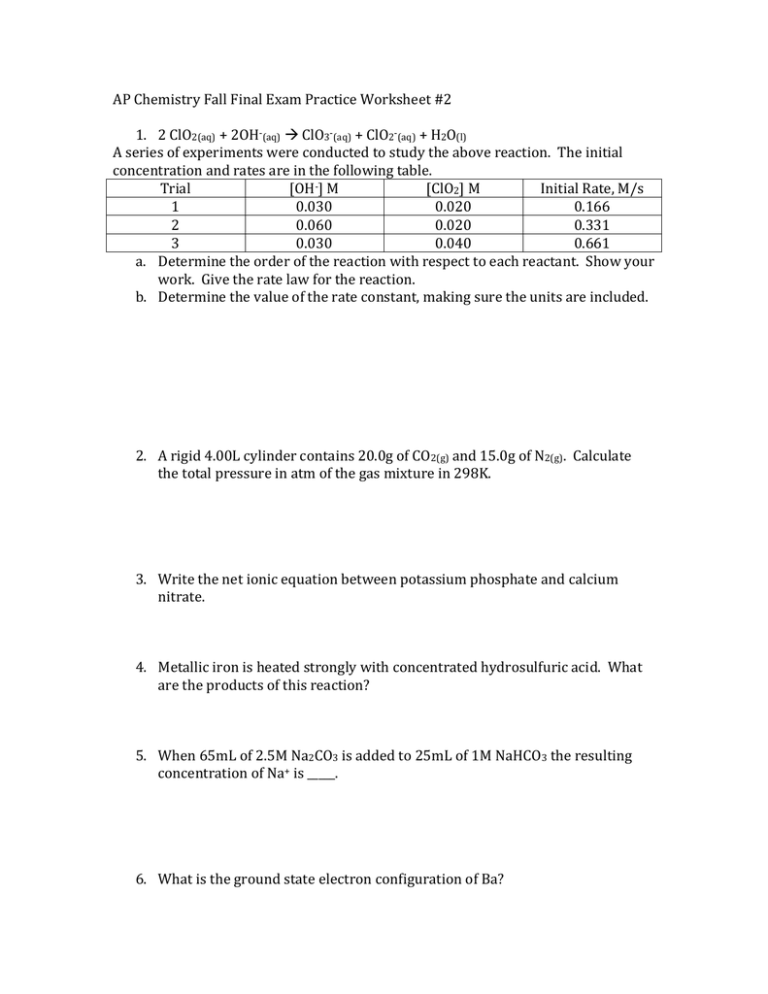
AP Chemistry Fall Final Exam Practice Worksheet #2 1. 2 ClO2(aq) + 2OH-(aq) ClO3-(aq) + ClO2-(aq) + H2O(l) A series of experiments were conducted to study the above reaction. The initial concentration and rates are in the following table. Trial [OH-] M [ClO2] M Initial Rate, M/s 1 0.030 0.020 0.166 2 0.060 0.020 0.331 3 0.030 0.040 0.661 a. Determine the order of the reaction with respect to each reactant. Show your work. Give the rate law for the reaction. b. Determine the value of the rate constant, making sure the units are included. 2. A rigid 4.00L cylinder contains 20.0g of CO2(g) and 15.0g of N2(g). Calculate the total pressure in atm of the gas mixture in 298K. 3. Write the net ionic equation between potassium phosphate and calcium nitrate. 4. Metallic iron is heated strongly with concentrated hydrosulfuric acid. What are the products of this reaction? 5. When 65mL of 2.5M Na2CO3 is added to 25mL of 1M NaHCO3 the resulting concentration of Na+ is _____. 6. What is the ground state electron configuration of Ba?