kinetics
advertisement
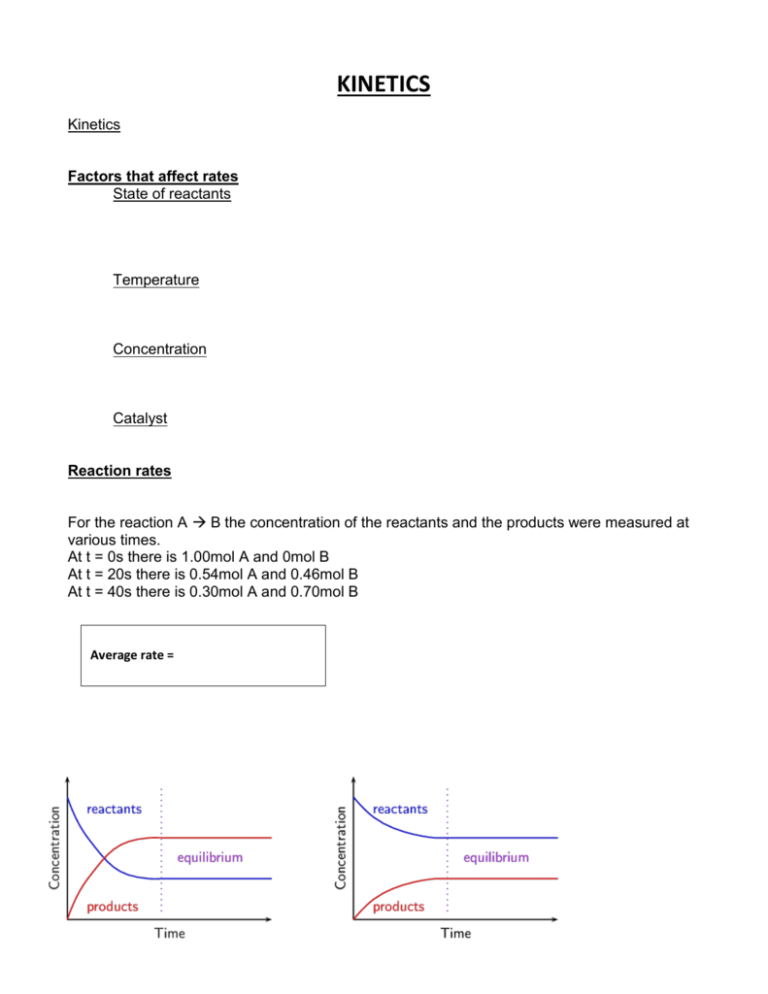
KINETICS Kinetics Factors that affect rates State of reactants Temperature Concentration Catalyst Reaction rates For the reaction A B the concentration of the reactants and the products were measured at various times. At t = 0s there is 1.00mol A and 0mol B At t = 20s there is 0.54mol A and 0.46mol B At t = 40s there is 0.30mol A and 0.70mol B Average rate = C H Cl(aq) + H O(l) C H OH(aq) + HCl(aq) 4 9 2 4 9 Reaction Rates and Stoichiometry C H Cl(aq) + H O(l) C H OH(aq) + HCl(aq) 4 9 2 4 9 1:1 ratio But what if it is not 1:1? 2 HI(g) H2(g) + I2(g) To generalize, for reaction aA + bB cC + dD Rate = 1. How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction: 2 O3(g) → 3 O2(g)? 2. If the rate at which O2 appears, Δ[O2]/ Δt, is 6.0 × 10–5 M/s at a particular instant, at what rate is O3 disappearing at this same time, –Δ[O3]/Δ t? Dependence of Rate on Concentration Rate Law NH4+ (aq) + NO2- (aq) N2 (g) + 2H2O (l) Rate = k[reactants]m Rate Constant Don’t forget units! Order of reaction Rate = k [NH4+] [NO2−] Order with respect to NH4+ = Order with respect to NO2− = Overall order = 22 = what happens to rate order Double the concentration 3. What are the overall reaction orders for the reactions: 2N2O5 4NO2 + O2 rate = k [N2O5] CHCl3 + Cl2 CCl4 + HCl rate = k [CHCl3][Cl2]1/2 H2 + I2 2HI rate = k [H2][I2] NO2 NO + ½O2 rate = k [NO2]2 overall order = overall order = overall order = overall order = 4. The initial rate of a reaction A + B → C was measured for several different starting concentrations of A and B, and the results are: Using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [A] = 0.050 M and [B] = 0.100 M. 5. For the reaction: 2NO(g) + O2(g) 2NO2(g) The following data was collected: Trial Initial [NO] Initial [O2] Rate of NO2formation 1 0.01 0.01 0.05 2 0.02 0.01 0.20 3 0.01 0.02 0.10 Determine the rate law and rate constant 6. The following data were measured for the reaction of nitric oxide with hydrogen: (a) Determine the rate law for this reaction. (b) Calculate the rate constant. (c) Calculate the rate when [NO] = 0.050 M and [H2] = 0.150 M The change of concentration with time Integrated rate laws 1st order 7. The decomposition of a certain insecticide in water follows first-order kinetics with a rate constant of 1.45 yr–1 at 12 °C. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5.0 × 10–7 g/cm3. Assume that the average temperature of the lake is 12 °C. a) What is the concentration of the insecticide on June 1 of the following year? b) How long will it take for the concentration of the insecticide to decrease to 3.0 × 10 –7 g/cm3? 2nd order Half Life For a 1st order process First Order Second Order Second Order Rate Laws Rate = - k [A] Rate = - k [A]2 Rate = - k [A] [B] Integrated Rate Laws 1 =kt + 1 [A]t [A]0 Complicated! ln [A]t - ln [A]0= - k t Graph ln [A] vs. t is linear 1 vs. t is linear [A] Half Life 0.693 = t1/2 k 1 = t1/2 k [A]0 Complicated ! Collision Theory An effective collision must have particles that collide with Activation energy PE diagrams Temperature and rate 8. Consider a series of reactions having the following energy profiles: Rank the reactions from slowest to fastest Reaction Mechanisms Overall reaction: A + 2B E + F A+BC C+BD DE+F Molecularity - Rate Law of an elementary step 9. It has been proposed that the conversion of ozone into O 2 proceeds by a two-step mechanism: a) Describe the molecularity of each elementary reaction in this mechanism. b) Write the equation for the overall reaction. c) Identify the intermediate(s). Multistep Mechanisms Rate Determining Step NO2 (g) + CO (g) NO (g) + CO2 (g) The rate law for the above reaction is found experimentally to be Rate = k [NO2]2 A proposed mechanism for this reaction is Step 1: NO2 + NO2 NO3 + NO (slow) Step 2: NO3 + CO NO2 + CO2 (fast) 10. The decomposition of nitrous oxide, N2O, is believed to occur by a two-step mechanism: a) Write the equation for the overall reaction. b) Write the rate law for the overall reaction. c) Identify any intermediates Fast Initial Step 2 NO (g) + Br2 (g) 2 NOBr (g) The rate law for the above reaction is found to be Rate = k [NO]2 [Br2] Because termolecular processes are rare, this rate law suggests a two-step mechanism. A proposed Step 1: NO + Br2 mechanism for this reaction is NOBr2 (fast) Step 2: NOBr2 + NO 2 NOBr (slow) Overall rate = But how can we find the concentration of an intermediate?? Assume fast step is in equilibrium! Now plug back into overall rate law 11. Ozone reacts with nitrogen dioxide to produce dinitrogen pentoxide and oxygen: The reaction is believed to occur in two steps: The experimental rate law is rate = k[O3][NO2]. What can you say about the relative rates of the two steps of the mechanism? Catalysts Catalysts increase the rate of a reaction by