1. Define an organic compound 2. Understand why
advertisement

1401 L7 Molecules of Life
Aims:
1. Define an organic compound
2. Understand why carbon is so important
3. Describe the basis of polymerisation
4. Identify the structure of carbohydrates, lipids, proteins & nucleic acids
5. Describe how this structure relates to the function of these molecules
Organic Compounds:
1. Inorganic (mineral) compounds are small, simple & relatively un-reactive
2. Organic compounds always contain carbon
3. Responsible for chemical reactions that make life possible
i) Some are water soluble
ii) Reactions take place in aqueous solutions
Importance of Carbon:
1. Chief component of organic structures
2. Ability to form long chains - from 2 → 1000’s atoms long
3. Many shapes - many different compounds with unique structures & functions
4. Some do not dissolve in water - useful building material
Organic Compounds:
1. Usually large molecules
2. Atoms usually joined by covalent bonds
3. Four main organic compounds:
i) Carbohydrates.
ii) Lipids.
iii) Proteins.
iv) Nucleic acids.
Monomers & Polymers:
1. Organic molecules combine to form larger ‘macromolecules’
2. Most macromolecules are ‘polymers’ - Poly – many, mers – parts
3. Polymers constructed from many identical / similar ‘monomers’ - Mono – one
Example: Whole chain is a polymer
Each link is a monomer
Individual monomers joined together form polymer
1
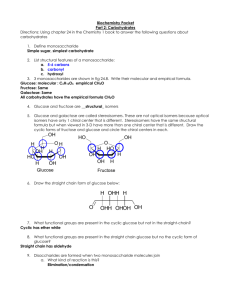
Carbohydrates:
1. Contain carbon, hydrogen & oxygen in ratio approx 1:2:1 (CH2O)
2. Often abbreviated to CHO - Include sugars, glycogen, starches & cellulose
3. Make up 2-3% of your body mass
4. Major source of energy in humans - also cell walls, exoskeletons (chitin) etc.
5. Dependant on their chemical structure
i) Mono/saccharides - (single sugar)
ii) Di/saccharides - (two sugars)
iii) Poly/saccharides - (many sugars)
Mono/saccharides:
1. Simple sugars
{-ose always means sugar}
2. Include:
i) Glucose (blood sugar)
ii) Fructose (fruit sugar)
iii) Galactose (milk sugar)
iv) Deoxyribose (in DNA)
v) Ribose (in RNA)
3. Building blocks of more complex carbohydrates
Glucose:
i) C6H12O6
ii) Hexose sugar
iii) Important fuel for humans
iv) Easily dissolves in water
Di/saccharides:
1. Main disaccharides include
i) Sucrose (Table sugar) = glucose + fructose
ii) Lactose (Milk sugar) = glucose + galactose
iii) Maltose (malt sugar) = glucose + glucose
Joining Monomers (monosaccharides)
2
Poly/saccharides:
1. Also called complex carbohydrates
2. Contain more than two monosaccharides
3. Important polysaccharides include:
i) Glycogen = fuel store in animals
ii) Starch = fuel store in plants
iii) Cellulose = plant cell wall component, not digested by humans = fibre
4. Chains straight or branched
5. Usually insoluble in water & do not taste sweet
6. Major energy source & store - Broken to single glucose
molecules for energy by digestion processes
Branched Chains of Glycogen
Glycogen stored in tissues e.g., liver & skeletal
muscle
Chains broken down & monomers released when
energy needed
Lipids:
1. Includes fats, oils & waxes
2. Constitutes large proportion of body weight
i) 12-18% males
ii) 18-24% females
3. Mostly insoluble in water
4. Major structural component in cells
5. Large energy store
6. Lipids contain around twice the energy of carbohydrate & protein per gram
Food Group
Avg. energy (kcal/g)
Protein
4.05
Carbohydrate
4.03
Lipid
8.93
Triglycerides:
1. A main lipids in body
2. From: three x fatty acids + glycerol
3. Solid or liquid at room temperature depends on fatty acid structure:
i) Saturated fats usually solid (e.g., butter)
ii) Unsaturated & polyunsaturated fats liquid (e.g. vegetable oils)
3
Triglyceride
Phospholipid
Lipid function:
1. Provide insulation, protection & cushioning
2. Valuable vitamin source – A, D, E & K
3. XS energy from carbohydrate, protein & fat from diet stored as triglycerides
4. Body - unlimited ability to store fat, useful for energy storage, not necessary in modern
lifestyle = obesity
Proteins:
1. Most NB & abundant component of human body
2. Contribute 12-20% body weight
3. Constructed from amino acids, usually 1000 to 100,000
4. Amino acids joined by covalent bonds called ‘peptide bonds
Protein Functions:
1. Structural support
2. Movement via contractile proteins
3. Transport via transport proteins
4. Buffering – maintaining pH
5. Catalytic via enzymes
6. Control & regulation via hormones
7. Defence via antibodies (IgG mum/bub, IgE histamine, IgM 1st, IgA tears)
4
Protein Structure:
1. Amino acids join to form ‘polypeptide chain’
2. Amino acid structure:
i) contain 5 key components
ii) Central carbon atom
iii) Hydrogen atom
iv) Amino group (NH2)
v) Carboxylic group (COOH)
vi) Variable group (R)
Amino Acids
Polypeptide Chain Formation
1. Polypeptide chain: peptide bonds form between amino & carboxyl groups
5
Protein Structural Levels:
1. Proteins rarely exist as simple polypeptide chains
2. Four levels of protein structure
i) Primary
ii) Secondary
iii) Tertiary
iv) Quaternary
Primary Structure:
Secondary Structure
Tertiary Structure
Quaternary Structure
6
Nucleic Acids:
1. Very large molecules
2. Two forms of nucleic acid
3. Deoxyribonucleic acid (DNA):
i) Inherited genetic material (genes)
ii) Regulates most of biochemical activity
4. Ribonucleic acid (RNA):
i) Copies DNA for protein synthesis
ii) Involved in manufacture of polypeptide chains
5. Constructed from nucleotides
i) Pentose sugar (ribose or deoxyribose)
ii) Phosphate group
iii) Nitrogenous base:
i) Adenine
ii) Guanine
iii) Cytosine
iv) Thymine (DNA only)
v) Uracil (RNA only)
DNA Structure:
Nucleotide Structure:
7
Summary:
1. Organic compounds contain Carbon
2. Four main organic molecules are carbohydrates, lipids, proteins & nucleic acids
3. Create polymer chains from repeating monomers
4. Provide structure, energy, genetic material & cellular regulate function
8