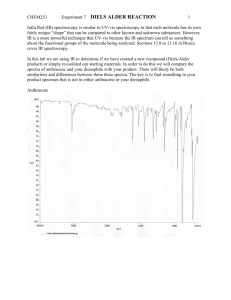
Smith Sections 21.10, 21.4A, 16.6, 16.15 Pre-lab
advertisement

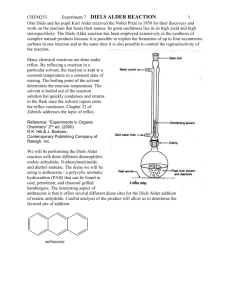
EXPERIMENT 4 THE WITTIG REACTION. SYNTHESIS OF TRANS-9-(2-PHENETHYLETHENYL)ANTHRACENE AND CHEMILUMINESCENT REACTION ITS USE IN A Reading Assignment: Smith Sections 21.10, 21.4A, 16.6, 16.15 Pre-lab Questions, Week 1: 1. Prepare a flow diagram showing the procedure for isolation and purification of the product. Where does the triphenylphosphine oxide byproduct end up? 2. Show how the starting phosphonium reagent could be prepared. 3. Sodium hydroxide is a very mild base for deprotonation of the phosphonium salt. For methyl triphenylphosphonium chloride, n-butyllithium is the typical base of choice. Why is possible to use NaOH here? (To state it another way, why is this phosphonium salt so acidic?) The Wittig reaction is a reliable and general synthetic method for the conversion of a ketone or aldehyde into an alkene. The active reagent in this reaction is a phosphonium ylid, which, because of its instability, is generated in situ (in the presence of the carbonyl compound that it will react with.) In this experiment, you will generate the phosponium ylid from a phosphonium salt using concentrated sodium hydroxide as the base. When the ylid is generated in the presence of 9-anthraldehyde, trans-9-(2-phenylethenyl)anthracene is formed. CHO NaOH P H P C C H Cl trans-9-(2-Phenylethenyl)anthracene While the details of the reaction mechanism are dependent on several variables, it is accepted that the carbanion attacks the carbonyl group to yield an alkoxide-phosphonium intermediate that collapses to the four-membered oxaphosphetane ring. This four membered ring is unstable and spontaneously undergoes fragmentation to give the alkene and triphenylphosphine oxide. CHEM M25LB/H52LB Experiment 4 1 Page Ph Ph P Ph R R'CHO Ph Ph P Ph Ph Ph Ph P O R R' R R Ph3P O + R' R' O phosphonium ylid oxaphosphetane intermediate trans-9-(2-Phenylethenyl)anthracene produces a green fluorescence when combined with the chemiluminescent compound Cyalume. Chemiluminescence is the process in which light is produced by a chemical reaction. Most exothermic reactions produce energy in the form of heat, but a few produce light and release little or no heat. Chemiluminescence is what causes fireflies to glow and light sticks to light. Chemiluminescent processes occur when the energy given off in a chemical reaction produces molecules in an electronically excited state. As these molecules drop down to the ground state, the light emitted is visible. Many such reactions occur in nature where they are referred to a biolumenescence. Lightsticks contain a solution of Cyalume and a fluorescer. Bending the light stick activates it (by breaking a small glass sealed tube filled with hydrogen peroxide, which mixes with the Cyalume/fluorescer solution) to give off a bright glow in a variety of colors. The different colors are created by using different fluorescers. The observed color depends on the structure of the fluorescer. In the light producing reaction, the ester of Cyalume reacts with hydrogen peroxide to produce a peroxyoxalic acid. Intramolecular displacement generates a small intermediate molecule which has been suggested to have the structure, A (this small molecule remains elusive, and has not yet been detected by IR, CNMR, or MS.) A then transfers its energy to the fluorescer, which is excited to the singlet excited state. When the excited state relaxes back down to the ground state, it releases light. Cl Cl O O Cl OC CO Cl Cl Cl H2O2 Cl O O OC COOH Cl Cl Bs(2,4,6-trichlorophenyl)oxalate Cyalume O Cl O + 2 Cl OH O O A CHEM M25LB/H52LB Experiment 4 2 Cl Page O O O O H C C H + charge transfer complex A trans-9-(2-Phenylethenyl)anthracene 2-CO2 + trans-9-(2-Phenylethenyl)anthracene* (singlet excited state) hn + trans-9-(2-Phenylethenyl)anthracene (ground state) Safety Precautions: Sodium hydroxide is extremely damaging to the eyes and skin. Keep organophosphorous compounds off the skin. Avoid skin contact with DMF. It can go through gloves, carrying with it residues of other compounds that are on the gloves onto your skin, where it is readily absorbed. You must wear gloves and eye protection at all times. Part A: Synthesis of trans-9-(2-phenylethenyl)anthracene: To a 10 mL round bottom flask equipped with a small stir bar, add benzyl triphenylphosphonium chloride (200 mg, 0.514 mmol), 9-anthraldehyde (115 mg, 0.558 mmol), and N,N-dimethylformamide (DMF) (0.5 mL). While vigorously stirring the solution, carefully add 50% NaOH (50% w/w) (0.25 mL) dropwise. If necessary, rinse any solid of the sides of the walls with additional DMF (about 5 drops). Continue the vigorous stirring for 30 minutes. At the end of the stirring period, add 4 mL of a 1:1 mixture of 1-propanol/H2O to precipitate the product. Collect the crude product by vacuum filtration, and TLC using 8:2 Hex:Ethyl Acetate. The product should be bright fluorescent blue, and the starting material, if present, yellow when irradiated with long-wave radiation. Transfer to a 10 mL Erlenmeyer and recrystallize using about 4 mL 1-propanol. The product crystallizes as thin yellow plates, mp 131 –132°C. Calculate the yield of the purified trans-9-(2-phenylethenyl)anthracene and obtain a melting point and UV spectrum. A typical concentration for the UV spectrum is 0.050 g/L. In your laboratory writeup, include and interpret the UV spectra for trans-9-(2phenylethenyl)anthracene. CHEM M25LB/H52LB Experiment 4 3 Page Part B: Chemiluminescent Experiment In a scintillation vial, dissolve 50 mg Cyalume [bis(2,4,6-trichloro)oxalate] and 3mg of the trans-9-(2-phenylethenyl)anthracene synthesized in Part A in 5 mL diethylphthalate by warming in a hot water bath. In anther scintillation vial, mix 0.2 mL of 30% hydrogen peroxide with 5 mL diethyl phthalate, cap, and shake to mix. Add the peroxide suspension dropwise to the Cyalume solution in a darkened hood. Warm the solution and then cool the solution to see the effect that this has on the rate of reaction. If additional trans-9-(2-phenylethenyl)anthracene is available, add 2 mg more along with 0.2 mL additional peroxide. What is the effect of adding the additional fluorescer and peroxide? Submit the remaining trans-9-(2-phenylethenyl)anthracene in a scintillation vial, and the capped vial with your chemilumenescent reaction mixture to your TA. References: 1. The procedure for Part A adapted from: C. Jaworek, S. Iacobucci. J. Chem Ed. 2002, 79(1), 111. 2. The procedure for Part B is adapted from: Kenneth L. Williamson, Organic Experiments, 9th Ed. Houghton Mifflin Company, 2004, p. 558-563. Post-lab Questions: 1. Who received the Noble Prize for the discovery of the Wittig reaction? 2. The Wittig reaction usually gives a mixture of cis and trans isomers, yet in this reaction, only the trans isomer is formed. The stereochemistry of product is set in the first step of the reaction of the ylid with the aldehyde. Draw the intermediate oxaphosphetane that leads to the trans-alkene geometry, and the one that would lead to the cis - alkene geometry, showing the stereochemistry at all of the chiral centers. Can you suggest a reason for the fact that only the trans alkene is formed? 3. trans-9-(2-phenylethenyl)anthracene can be easily distinguished from cis-9-(2phenylethenyl)anthracene by looking at the HNMR. Indicate how you could tell the difference between the two by HNMR. 4. It is often possible to make an alkene from two different combinations of a carbonyl group and a phosphonium salt. What is the other combination of carbonyl compound and phosphonium ylide that could be used to make the product in this reaction? CHEM M25LB/H52LB Experiment 4 4 Page