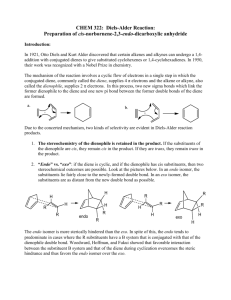
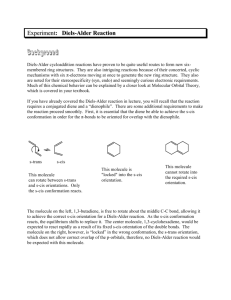
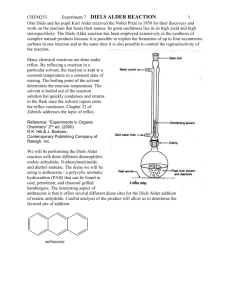
The Diels-Alder Reaction
advertisement

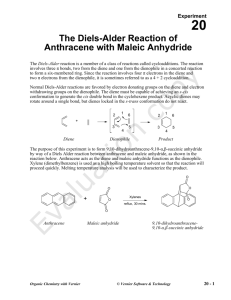
Page 1 of 4 The Diels-Alder Reaction Reaction of Maleic Anhydride with Anthracene Otto Diels (1876-1954) Kurt Alder (1902-1958) The Diels-Alder reaction is one of the most versatile reactions in organic synthesis. It is named for Otto Diels and his research student, Kurt Alder, who discovered and developed the reaction in the late 1920’s. The importance of this reaction in understanding pericyclic reactions and as a tool in synthetic organic chemistry led to their award of a Nobel Prize in 1950. The Diels-Alder reaction is a concerted (one step) reaction between a diene and ‘dienophile’ to form a cyclohexene ring as shown in Figure 1. Because the reaction 4 + 2 Figure 1 involves 4 electrons in the diene and 2 electrons from the dienophile, it is sometimes referred to as a 4 + 2 cycloaddition. Low molecular weight dienes typically are volatile, unpleasant smelling substances. This laboratory exercise uses anthracene as the diene to avoid this unpleasant personality trait. Anthracene has a relatively high molecular weight, and therefore a low vapor pressure and odor. However, it is classified as an aromatic compound. You will study aromatic compounds in more detail later in the semester; aromatic compounds are significantly stabilized by resonance and therefore are relatively unreactive as dienes in the Diels-Alder reaction. We will compensate for the low reactivity of anthracene by using a highly reactive dienophile, maleic anhydride (II) (Why is it highly reactive?). The reaction will also be run at high temperature to increase the rate. The reaction is shown JPN 7-9-10 Page 2 of 4 anthracene maleic anhydride 9,10 dihydroanthracene-9,10-succinic acid anhydride Figure 2 in Fig 2. The new cyclohexene ring is highlighted in red. Note that the numbers are used to identify the carbon atoms involved in the reaction and do not correspond to the nomenclature numbering system. The nomenclature system used to name the product will not be covered here. The reaction mechanism is shown in Fig (3). Because of the ring structure of anthracene, it is a bit awkward to draw the ‘arrow pushing’ mechanism. However, recall that the dienophile approaches the diene from above or below the system of the diene. In the mechanism below, the diene portion of anthracene and dienophile double bond are highlighted. The result of the arrow pushing mechanism results in bond formation between carbons 1-3 and carbons 2-6 as labeled in Fig 2. The double bond in the product is now between carbons 4-5. Product Figure 3 As part of your pre-lab activities, include a table of the relevant properties of the starting materials and solvents. You should also have a copy of the IR spectra of anthracene and maleic anhydride. JPN 7-9-10 Page 3 of 4 Procedure: 1. Weigh 775-825mg of anthracene and 390-410mg maleic anhydride in a 25mL round bottom flask. Make sure you record the weight of each substance to 0.001g. (You will, of course, determine which substance is in molar excess here) 2. Add 10mL of xylene (dimethylbenzene, generally a mixture of isomers) and a boiling stone to the flask 3. Clamp the flask securely and attach a reflux condenser and drying tube filled with calcium chloride to the apparatus. Maleic anhydride is water sensitive and the drying tube will help prevent water in the atmosphere from entering the reaction flask. 4. Heat the reaction mixture with a heating mantle to reflux. Because of the relatively high concentration of substrates in the reaction, the system will maintain a reflux temperature of 180-200oC, considerably higher than the normal boiling point of xylene. 5. Reflux for 30 minutes and record your observations. 6. After 30 minutes, allow the temperature to cool to room temperature and then place the flask in an ice bath for 10 minutes to complete crystallization. 7. Collect the solids by suction filtration 8. Wash the solids with 3mL of ethyl acetate and suck dry for 5 minutes. 9. Place the solids in a drying oven at 90-1000C until dry (how will you know?) and record the weight. 10. Obtain a melting point and IR of your product. Post Lab: (Results and Conclusion) At a minimum Be sure to calculate your per cent yield and clearly identify the limiting reagent. Use your spectral data to speculate on the presence of any starting material that may still be present in your product. Describe what factors (if any) may cause you to achieve less than 100% yield. Based on your experience with this reaction, draw the product of the following related reactions: JPN 7-9-10 Page 4 of 4 Enrichment: (These need not be mentioned in your report, but are for practicing your ability to view the bonds formed in Diels-Alder reactions.) The following Diels-Alder reactions have been used in recent synthetic processes. The diene and dienophile systems are highlighted in red. These reactions use more complex starting materials than typically seen in undergraduate courses, but you should be able to trace the bond forming and breaking patterns JPN 7-9-10