Works Cited
advertisement

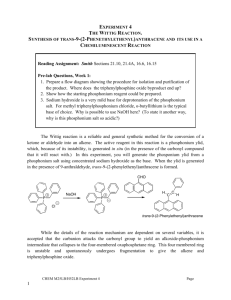
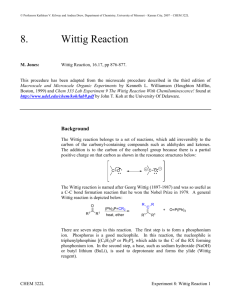
THE WITTIG REACTION SHAUN LYNN J9106574 Abstract Carbon to carbon double bonds can be created by the Wittig Reaction. In this reaction, a nucleophilic ylid attacks a carbonyl group, and the ringed intermediate collapses to form a double bond with the acyl carbon in place of the oxygen. In this experiment, trans-9-(2phenylethenyl)anthracene was synthesized from 9-anthraldehyde and benzyltriphenylphosphonium chloride via the Wittig Reaction. The goal of this experiment was to obtain a high percent yield and purity for the synthesis of trans-9-(2phenylethenyl)anthracene. A percent yield of 48.68% was obtained for the synthesized product, and the observed melting point range value was 2°C lower than the literature value range. The results supported that an efficient synthesis had taken place. Introduction Discovered in 1954 by Georg Wittig (for which he was awarded the 1979 Nobel Prize in Chemistry), the Wittig reaction is a nucleophilic substitution reaction, used in the synthesis of alkenes. The Wittig reaction is the overall substitution of a C=O bond to a C=C bond. The double bond forms specifically at the location of the original ketone or aldehyde (McMurry, 2008). The aldehyde/ketone will react with a species known as a phosphonium ylid, which is a neutral molecule with positive and negative charges on adjacent atoms; the positive charge in this case will be situated on the phosphorus. The ylid is prepared via a two step process: To begin, an SN2 reaction between triphenylphosphine and an alkyl halide followed by treatment with a strong base. The nucleophilic carbon of the Wittig reagent adds to the electrophilic carbon in the polar carbonyl group. Electrons from the C=O π bond will then be used to form a σ bond to the positive phosphorus atom. This creates a cyclic intermediate called an oxaphosphetane (Carey, 2007). Decomposition of the intermediate by breaking the C-P and C-O σ bonds leads to the formation of the C=C π bond of the alkene and triphenylphosphine oxide. (Carey, 2007) Shaun Lynn Chemical reactivity J9106574 Aim To prepare a carbon – carbon double bond by converting a carbonyl compound into an alkene via the Wittig reaction, the mechanism of which can be seen below. The removal of a proton from benzyltriphenylphosphonium chloride will create an ylide, which will then attack the 9-anthraldehyde carbonyl group. The disintegration of the 4ringed intermediate will then produce the desired end product. Shaun Lynn Chemical reactivity J9106574 Hazards Benzyltriphenylphosphonium chloride - Toxic Dichloromethane – Toxic, suspect carcinogen Sodium hydroxide – causes severe burns 9-anthraldehyde – avoid direct with skin and eyes, avoid inhalation. Method Benzyltriphenylphosphonium chloride (1.90g) [balance N0017425] and 9-anthraldehyde (1.19g) were weighed and added to a round bottomed flask (25cm³). Dichloromethane (6.0cm³) was measured using a measuring cylinder and added to the flask. The flask was then stirred using a magnetic stirrer at high speed whilst adding sodium hydroxide (50% w/v 2.6cm³) dropwise using a Pasteur pipette. After complete addition of the sodium hydroxide, the mixture was left to stir for a further 30 minutes before being transferred to a separate flask, using water (20cm³) and dichloromethane (20cm³) to complete the transfer. The mixture was shaken and left to settle, the organic layer was removed to another flask and the aqueous layer was extracted once more with dichloromethane (10cm³) and then combined with the previous organic extract. The combined organic layers were dried with anhydrous calcium chloride pellets, transferred o a conical flask (100cm³) and evaporated to dryness on a steam bath. The product was then recrystallised from 2-propanol, the melting point and IR spectrum of the recrystallised product was then recorded. Discussion Mass of product obtained = 0.925g Melting point range = 126.1°C – 128.1°C According to Dictionary of organic compounds, 6th edition, Chapman and Hall, London, 1996 the melting point range for 9-(2 phenylethenyl)anthracene is 130°C – 132°C. As the melting point range is only 2°C lower than the cited literature value, this suggests that the synthesised product was indeed 9-(2-phenylethenyl)anthracene with a very high purity. From an examination of the molecular structure of 9-(2 phenylethenyl)anthracene, the formation of the Z-Cis 9-(2-phenylethenyl)anthracene is highly unlikely due to steric hindrance, the size of the two groups at either side of the double bond are too large. Shaun Lynn Chemical reactivity J9106574 Therefore the stereoisomer formed is E-Trans 9-(2 phenylethenyl)anthracene exclusively. Yield calculations Mr of benzyltriphenylphosphonium chloride [BTP] C25H22PCl = 388.869 (1.90g) 9-anthraldehyde C15H10 O = 206.2293 (1.19g) 1 BTP = (1.90𝑔 𝑥 (388.869 ) = 0.0048859 𝑚𝑜𝑙𝑒𝑠 1 9-anthraldehyde = 1.19𝑔 𝑥 (206.2293 ) = 0.00577 𝑚𝑜𝑙𝑒𝑠 The limiting reagent is therefore Benzyl triphenylphosphonium chloride. 0.0048859𝑚𝑜𝑙𝑠 𝑥 ( 388.869 ) = 1.90𝑔 1 The theoretical yield of E-Trans 9-(2 phenylethenyl)anthracene is 1.90g Percentage yield = Shaun Lynn 0.925𝑔 1.90 𝑥 100 = 𝟒𝟖. 𝟔𝟖% Chemical reactivity J9106574 Shaun Lynn Chemical reactivity J9106574 IR interpretation The black ring at 3030.52 indicates the presence of sp 2 hybridised C-H bonds, as well as C-H bonds on the conjugated system. The Blue circle at highlights non aromatic C=C bonds at around 1674.96 and aromatic C=C bonds at around 1575.80 The orange circle indicates the presence of a trans alkene, which supports the fact that the product is in fact E-Trans 9-(2 phenylethenyl)anthracene. The green circle highlights aromatic C-H bending. WORKS CITED Carey, F. S. (2007). Advanced Organic Chemistry: Reactions and Synthesis (5th Edition ed.). London: Springer. Hunt, I. (2011, 04 10). The Wittig reaction. Retrieved 04 10, 2011, from University of Calgary: http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch17/ch17-3-2-3.html Trippet, S. (2011, 04 10). The Wittig Reaction. Retrieved http://www.iupac.org/publications/pac/1964/pdf/0902x0255.pdf 04 10, 2011, from IUPAC.org: Unknown. (2011, 04 15). Infrared spectroscopy correlation table. Retrieved 04 15, 2011, from Wikipedia, the free encyclopedia: http://en.wikipedia.org/wiki/Infrared_spectroscopy_correlation_table Dictionary of organic compounds, 6th edition, Chapman and Hall, London, Volume 3(& Volume 6), 1996 McMurry, J. (2008). Organic Chemistry. J. McMurry, organic Chemistry . London: Thompson Brooks/Cole. Wittig Reaction: The Synthesis of trans-9-(2-Phenylethenyl)anthracene Revisited, C. Jaworek and S. Iacobucci Department of Chemistry, Tufts University, Medford, MA 02155 J. Chem. Educ., 2002, 79 (1), p 111, DOI: 10.1021/ed079p111 ,Publication Date (Web): January 1 2002 Shaun Lynn Chemical reactivity J9106574