Diels-Alder Reaction: IR Spectroscopy Lab Experiment
advertisement

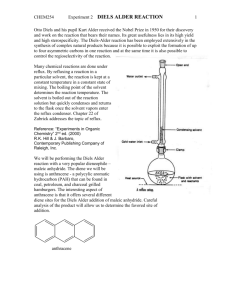
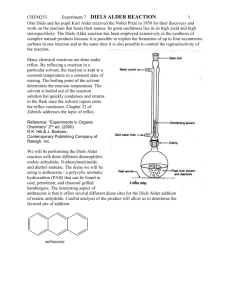
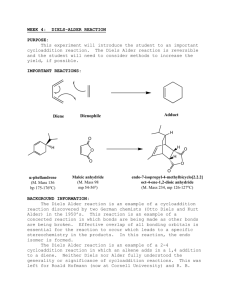
CHEM253 Experiment 7 DIELS ALDER REACTION 1 Infra Red (IR) spectroscopy is similar to UV-vis spectroscopy in that each molecule has its own fairly unique “shape” that can be compared to other known and unknown substances. However, IR is a more powerful technique that UV-vis because the IR spectrum can tell us something about the functional groups of the molecule being analyzed. Sections 13.8 to 13.16 in Bruice cover IR spectroscopy. In this lab we are using IR to determine if we have created a new compound (Diels-Alder product) or simply re-isolated our starting materials. In order to do this we will compare the spectra of anthracene and your dienophile with your product. There will likely be both similarities and differences between these three spectra. The key is to find something in your product spectrum that is not in either anthracene or your dienophile. Anthracene CHEM253 Experiment 7 maleic anhydride DIELS ALDER REACTION 2 CHEM253 Experiment 7 N-phenyl maleimide DIELS ALDER REACTION 3 CHEM253 Experiment 7 diethyl malonate DIELS ALDER REACTION 4 CHEM253 Experiment 7 DIELS ALDER REACTION anthracene + maleic anhydride product - student sample 5 CHEM253 Experiment 7 DIELS ALDER REACTION anthracene + N-phenyl maleimide product - student sample 6 CHEM253 Experiment 7 DIELS ALDER REACTION anthracene + diethyl malonate product - student sample 7