Lab 10 Enzymes
advertisement

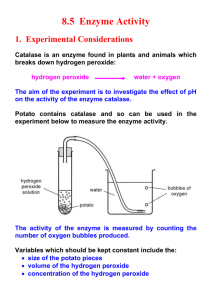
Lab10 Enzymes and Chemical Reactions Introduction: Part One In this lab, we will study the action of enzymes. Enzymes are specialized proteins that serve to lower the activation energy required to make cellular reactions occur. Activation energy is the energy required to start a reaction. For example, a ball cannot roll down a flight of stairs without you pushing it. In the same way cellular reactions cannot begin without the activation energy. Enzymes greatly speed up chemical reactions that would otherwise occur too slowly to sustain life. Check out the diagram at this link. Liver and other living tissues contain the enzyme catalase. This enzyme breaks down hydrogen peroxide, which is a harmful by-product of the process of cellular respiration if it builds up in concentration in the cells. If we use potato or other tissue containing this enzyme, we can use this to measure the relative influence of varying several different factors on the activity of enzymes in living tissue. In order to obtain energy and building blocks from food, the digestive system must break down proteins, fats and carbohydrates. Specific enzymes catalyze the reactions that breakdown each of these substances. In this lab, you will perform reactions involved in digestion of carbohydrates, lipids, and proteins and observe the results of these reactions. first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Introduction: Continued What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. The enzyme is not altered by the reaction. You have hundreds of different enzymes in each of your cells. Each of these enzymes is responsible for one particular reaction that occurs in the cell. In this lab, you will study an enzyme that is found in the cells of many living tissues. The name of the enzyme is catalase (KAT-uh-LAYSS); it speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen. The reaction is as follows: 2H2O2 ----> 2H2O + O2 This reaction is important to cells because hydrogen peroxide (H2O2) is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells. You will be using chicken or beef liver. It might seem strange to use dead cells to study the function of enzymes. This is possible because when a cell dies, the enzymes remain intact and active for several weeks, as long as the tissue is kept refrigerated. first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Demonstration Part A: Observe Normal Catalase Reaction 1. Your teacher will place 2 ml of the 3% hydrogen peroxide solution into a clean test tube. 2. A small piece of liver is cut (using forceps and scissors) and added add it to the test tube. A stirring rod is used to push it into the hydrogen peroxide. Measure the height of the bubbles and record the data in the data table on your answer sheet. 3. Answer the questions from part A on your answer sheet. Demonstration Part B: Is Catalase Reusable? 1. Again, your teacher will place 2 ml of 3% hydrogen peroxide solution into a clean test tube and add a small piece of liver. 2. Observe the reaction in the test tube and record how high the bubbles rise in the tube. 3. Your teacher will pour off the liquid into a second test tube. 4. Assuming the reaction is complete. Answer the following questions on your answer sheet: a. What is this liquid composed of? b. What do you think would happen if you added more liver to this liquid? c. Your teacher will help you test this. Observe the reaction rate and record how high the bubbles rise in the tube. d. Explain your results (Of what is the liquid composed?) 5. Observe your teacher add another 2 ml of hydrogen peroxide to the liver remaining in the first test tube. Is catalase reusable? (Respond on your answer sheet.) first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Demonstration Part C: What Tissues Contain Catalase? 1. To test for the presence of catalase in tissues other than liver, your teacher will place 2 ml of hydrogen peroxide in each of 3 clean test tubes and then add each of the three test substances to the tubes. a. To the first tube, add a small piece of potato. b. To the second tube, add a small piece of chicken. c. To the last tube, add a small piece of apple. 2. As each substance is added, record the reaction rate (height bubbles rise) for each tube. 3. On your answer sheet, document which tissues contained catalase. a. How you can tell? 4. Do some substances contain more catalase than others? a. How can you tell? first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Procedure: Design an Experiment to Test the Effect of Temperature and pH on Enzyme Activity 1. You and your partner will be teamed with another group in the class. Decide which group will test pH and which will test temperature. 2. Use your answer sheet to design your experiment. Remember to include safety procedures and have your instructor approve your design before beginning. Use the following information in your design: Materials available: Boiling water Timers Thermometers Ice NaOH (for making the solution basic) to test pH 10 HCl (for making the solution acidic) to test pH 3 Liver Hydrogen Peroxide Solution Test tube holder (for handling hot test tubes) Ruler (for measuring the size of your liver pieces & height bubbles rise) Test Tubes first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Procedure: Design an Experiment (continued) Safety Considerations for pH Experiments: Do not let acids or bases contact your skin or clothing. Swirl each test tube after adding each drop and measure the pH of each solution with pH paper. To do this, remove a drop or two of solution from a test tube using a clean glass stirring rod. Rinse your stirring rod and wipe dry before you dip it into each test tube. Place the drop on pH paper. pH measurements should be included in your data table. Safety Considerations for Temperature Experiments: Always use test tube holders when heating the test tube. Never hold the tube close to your face. Do not transfer hot glass to a cold container - it may burst! Never add cold material to a hot test tube. After approval of your experiment, and completion of all parts, record the data collected and answer the questions on your answer sheet. Don’t Forget to do your Homework! first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions References Holsinger, Rachel and Debbie Wheeler. “Lab 10: Enzymes.” Sayre School: Lexington, KY. January 2007. Muskopf, Shannan. “Enzyme Lab.” The Biology Corner. 8 Jan. 2007 <http://www.biologycorner.com/bio3/enzymelab.html>. first page email us glossary references 10. Lab10 Enzymes and Chemical Reactions Images bear-goggles. Photograph. 2004. Hot Dog and a Half. 8 Jan. 2007 <http://www.tigerlair.com/hotdogandahalf/bear-goggles.jpg>. Diagram of Digestive System. Digitally created image. 2002. WebMD. 8 Jan. 2007 <http://www.webmd.com/NR/rdonlyres/ 12443856-E8B5-42AE-84A9-E1E171CE9D8A.jpg>. Experiment. Drawing. Science Fair Resource Center, Efston Science, Inc. 8 Jan. 2007 <http://www.escience.ca/resource/resourceGFX/Experiment.gif>. POTATO. Photograph. California Institute of Technology, Department of Computer Science. Mika Nyström. 8 Jan. 2007 <http://www.async.caltech.edu/~mika/ potato/POTATO.jpg>. Test tubes with Hydrogen peroxide. Photograph. PowerPoints for McMurray & Fay Chemistry (4th Edition), Prentice Hall. 8 Jan. 2007 <http://wps.prenhall.com/wps/media/objects/602/616516/Media_Assets/ Chapter14/Text_Images/FG14_09.JPG>. first page email us glossary references 10.