Chapter 9
advertisement
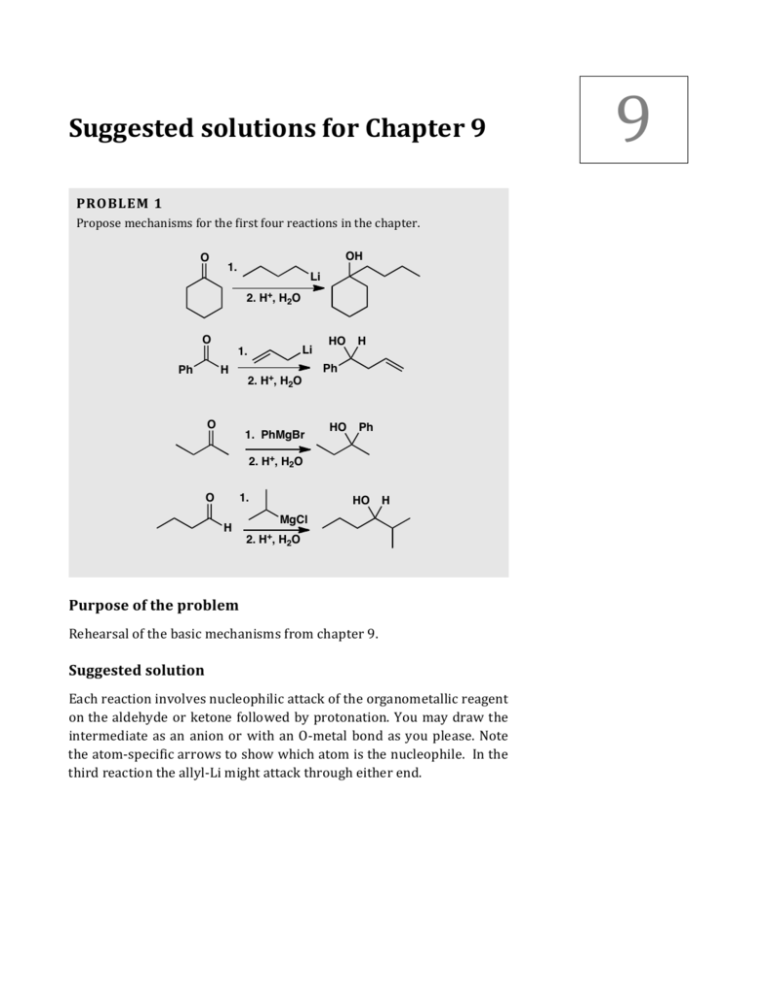
9 Suggested solutions for Chapter 9 PROBLEM 1 Propose mechanisms for the first four reactions in the chapter. O OH 1. Li 2. H+, H2O O Li 1. Ph H 2. H+, H2O O 1. PhMgBr HO H Ph HO Ph 2. H+, H2O 1. O H HO H MgCl 2. H+, H2O Purpose of the problem Rehearsal of the basic mechanisms from chapter 9. Suggested solution Each reaction involves nucleophilic attack of the organometallic reagent on the aldehyde or ketone followed by protonation. You may draw the intermediate as an anion or with an O-­‐metal bond as you please. Note the atom-­‐specific arrows to show which atom is the nucleophile. In the third reaction the allyl-­‐Li might attack through either end. 2 Solutions Manual to accompany Organic Chemistry 2e Li O O OH H+, H2O MgBr O BrMgO Ph Ph Ph HO H+, H2O Li O Ph LiO H H Ph Ph O O MgCl H H HO H+, H2O H H+, H2O R HO H R PROBLEM 2 What products would be formed in these reactions? 1. EtMgBr Ph H 2. Ph2CO 3. H+, H2O 1. Mg, THF A Br Br Cl 1. BuLi 2. CO2 3. H+, H2O 2. B O C Purpose of the problem The toughest test: predicting the product. The sooner you get practice the better. Suggested solution Though prediction is harder than explanation, you should get these right the first time as only the last one has a hint of difficulty. In the first example, the ethyl Grignard reagent acts as a base to remove a proton from the alkyne. Whether you draw the intermediate as an alkyne anion or a Grignard reagent is up to you. Notice that sometimes we give the protonation step at the end and sometimes not. This is the general practice among organic chemists and you may or you may not bother to draw the mechanism of this step. Solutions for Chapter 9 – Using Organometallic Reagents to Make C–C Bonds OH O Ph MgBr H Ph Ph Ph 3 Ph Ph Ph For the second example, just make the organometallic reagent and add it to the carbonyl group. Cyclobutanone is more electrophilic than many other ketones because of the strain of a carbonyl group in a four-­‐ membered ring. This time the protonation is shown. Mg, THF O Br BrMg H OH O The third example raises the question of which halogen is replaced. In fact bromine is more easily replaced than chlorine. Iodine is more easily replaced than either and fluorine usually does not react. Don’t be disappointed if you failed to see this. Cl Br BuLi Cl Li O Cl ■ This synthesis of carboxylic CO2 acids is on p. 190/1 of the textbook. C H O Cl CO2H PROBLEM 3 Suggest alternative routes to fenarimol different from the one in the textbook on p. 192. Remind yourself of the answer to problem 2 above. Purpose of the problem Practice in choosing alternative routes. Suggested solution Three aromatic rings are joined to a tertiary alcohol in fenarimol, so the alternatives are to make organometallic reagents from different aromatic compounds. Two aromatic compounds must be joined to form a ketone and the third added as an organometallic reagent. You will meet ways to make ketones like these in chapter 21. You’ll need the insight from problem 2 above to help you choose Br as the functional group to be lithiated or converted into a Grignard reagent. Here are two possible methods: 4 Solutions Manual to accompany Organic Chemistry 2e Cl Cl Cl BuLi N + or Mg Br fenarimol N M O Cl BuLi N + Br or Mg Cl fenarimol N M O Cl PROBLEM 4 Suggest two syntheses of the bee pheromone heptan-­‐2-­‐one. O Purpose of the problem Further exploration of the use of organometallic compounds. This time you’ll probably need the oxidation of alcohols from p. 194 of the textbook. Suggested solution There are of course many different solutions but the most obvious are to make the corresponding secondary alcohol and oxidise it. Two alternatives are shown here. O MeMgBr or MeLi H or OH CrO3 O O + H M Solutions for Chapter 9 – Using Organometallic Reagents to Make C–C Bonds PROBLEM 5 The antispasmodic drug biperidin is made by the Grignard addition reaction shown here. What is the structure of the drug? Do not be put off by the apparent complexity of the structure: just use the chemistry of Chapter 9. 1. Mg, Et2O Br 2. biperidin O N How would you suggest that the drug procyclidine should be made? HO N procyclidine Purpose of the problem Exercise in product prediction in a more complicated case and a logical extension to something new. Suggested solution A Grignard reagent must be formed from the alkyl bromide and this must add to the ketone. Aqueous acidic work up (not mentioned in the problem as is often the case) must give a tertiary alcohol and that is biperidin. Mg, Br Et2O HO MgBr N O N biperidin To get procyclidine, we must change both the alkyl halide and the ketone but the reaction is very similar. 5 6 Solutions Manual to accompany Organic Chemistry 2e MgBr Br Mg, Et2O + O N procyclidine









