CHEMICAL REACTIONS
advertisement

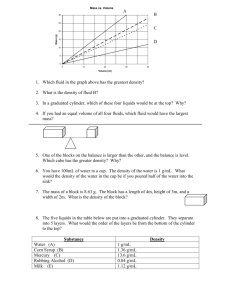
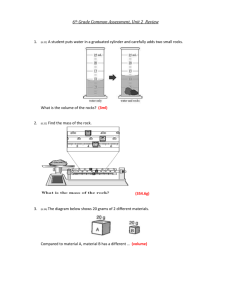
MEASUREMENTS EXPERIMENT 2 OBJECTIVE The objective of this experiment is to measure matter through various experimental techniques. The types of techniques covered will include: 1. Linear measurements using a metric rule. 2 Volumetric measurements using metric and graduated containers 3. Mass using triple beam balances 4. Density using combinations of the above EQUIPMENT AND CHEMICALS Graduated cylinder (100 ml) Gas bottle Beaker (400 ml) Triple beam balance Flask (250 ml) Metric ruler Assorted objects and unknowns Test Tube (6 in) DISCUSSION Chemistry is a science based on experimentation. These experimental results are determined by taking measurements. On relatively elementary experiments, the types of measurements used are in terms of mass, volume, length, temperature, and time. An understanding of these relationships is essential to the further development of one's skills in chemistry. The entire universe consists of matter and energy. Matter is defined as anything which has mass and occupies space. Mass and volume measurements are of primary importance in the study of matter. Mass is defined as the quantity of matter in a sample of any material. Volume is how much space this sample occupies. Metric System Chemistry, like most modern sciences, uses the metric system as the basis of measurement. The five principle units in the metric system are: the gram (mass); meter (length); liter (volume); centigrade (temperature); and seconds (time). Seconds are the same units as you are accustomed to, so they will no longer be discussed. 13 Mass Mass is measured using an instrument called a balance that is calibrated using standardized weights. Volume Volume is measured using either dimensional analysis (standardized rulers for solids) or volumetric analysis for liquids and irregularly shaped solids. Temperature Temperature is measured using a thermometer that has been standardized against water. Volume The liter is derived from the meter. A liter is nothing more than a space that has a height of 10 cm, a width of 10 cm, and a thickness of 10 cm. The liter is, therefore, equal to one thousand cubic centimeters (1000cm3). Density Density is a physical property (characteristic) of a substance and can be used to identify a substance. Density is defined as the ratio of the mass (grams) of a substance to the volume (cm3) occupied by the substance. When the density of a solid is given, the mass is expressed in grams and the volume in cm3. (1/1000 liter = 1 ml = 1 cm3 ) density = mass g g = = 3 volume ml cm DENSITY OF REGULARLY SHAPED OBJECTS If the substance is a regularly shaped object, the density can be determined using linear dimensional measurements and the mass. Example 1: Calculate the density of a block of wood that has a height of 10 cm, a width of 5 cm, and a thickness of 3 cm. The block was weighed on a triple beam balance and has a mass of 75 grams. Solution: The volume of the block of wood is equal to the height x width x length. volume = H x W x D volume = 10 cm x 5 cm x 3 cm = 150 cm 3 density = 75 g mass g g = = = 0.50 3 3 volume cm 150 cm cm 3 14 Example 2: Calculate the density of a triangular block of lead that has a mass of 900 grams, a height of 10 cm, a width of 5 cm, and a thickness of 3 cm. Solution: The volume of a triangle is equal to the height x width x length divided by 2. (a triangle is l/2 the size of a cube). volume = H xW x D 2 volume = 10 cm x 5 cm x 3 cm = 75 cm 3 2 density = 900 g mass g g = = = 12.0 3 3 volume cm 75 cm cm 3 Example 3: Calculate the density of a gold cylinder that has a height of 30 cm, a diameter of 3 cm, and a mass of 4091 gm. Solution: The volume of a cylinder is equal to the height times the radius squared times pi. The radius is 1/2 the diameter and π = 3.14 volume = π r 2 h 3 cm diameter radius = r = = = 1.5 cm 2 2 volume = 3.14 x 1.5 cmx x 1.5 cm x 30 cm = 212 cm 3 density = 4091 g mass g g = = = 19.3 3 3 volume cm 212 cm cm 3 DENSITY OF IRREGULARLY SHAPED OBJECTS Immersing the object in water and measuring the volume of liquid displaced can determine the volume of irregularly shaped objects. Example 4: Calculate the density of a toy car that has a mass of 500 grams. Solution: Immersing the car in water raised the volume of water from 200 ml to 450 ml. volume = final volume − initial volume = 450 ml − 200 ml = 250 ml density = 500 g mass g g = = = 2.0 volume ml 250 ml ml 15 PROCEDURE PART A - ACCURACY IN LIQUID MEASUREMENT The accuracy of any measurement is limited to one's ability to read the measurement. Many times, one has to estimate a reading when it is between two calibration marks. The larger the volume between the calibrated-marks, the greater the possibility of error when determining the volume. You will compare the measured volumes of water using various calibrated glassware. As a rule, the smaller the increments between marks, the greater the accuracy when estimating the volume. For example, a 100 ml graduated cylinder is calibrated in 100 one-milliliter marks. You can easily measure 1 ml. A 400 ml beaker is calibrated in 50 ml marks. A small miscalculation in the reading can result in a larger volume difference. To read any volume of liquid, we use a reference point on the liquid called a meniscus. This is the lowest point of the curve on the surface of a liquid. Look at the surface so that your eye is level with the meniscus and read the mark. 1. Using a 400 ml beaker, fill the beaker to the 200 ml mark with water. 2. Transfer this water to a 100 ml graduated cylinder in smaller increments (parts). What is the difference between the volumes determined in the graduated cylinder and the 200 ml in the beaker? 3. Using a 250 ml flask, fill to the 200 ml mark with water. 4. Transfer this water to a 100 ml graduated cylinder in smaller increments (parts). What is the difference between the volume determined in the graduated cylinder and the 200 ml in the beaker? 16 5. Fill a 6 inch test tube with water to the top, and measure the volume of water in the test tube by transferring the water in the test tube to a 100 ml graduated cylinder. 6. Fill a gas bottle with water to the top, and measure the volume of water using a 100 ml graduated cylinder. PART B - THE DENSITY OF A LIQUID The density of a liquid can be calculated by weighing a known volume of the liquid. 1. Weigh a dry 100 ml graduated cylinder. Record this mass in grams (g) on your Report Sheet. 2. Add approximately 90 ml of water to the cylinder. Read the volume as accurately as possible and record this volume in milliliters (ml) on your Report Sheet. 3. Weigh the 100 ml graduated cylinder that contains the water. Record the weight in grams (g) on your Report Sheet. 4. Calculate the density of water in g/ml. Unknown Liquid 1. Obtain an unknown liquid from your instructor. 2. Record the unknown number on your Report Sheet. 3. Weigh a dry 100 ml graduated cylinder. Record this mass in grams (g) on your Report Sheet. 4. Add approximately 90 ml of the unknown liquid to the cylinder. Read the volume as accurately as possible and record this volume in milliliters (ml) on your Report Sheet. 5. Weigh the 100 ml graduated cylinder with the water. Record the weight in grams (g) on your Report Sheet. 6. Calculate the density of the unknown in g/ml. PART C - THE DENSITY OF AN IRREGULARLY SHAPED OBJECT The density of an irregularly shaped object can be measured by using the displacement of water to determine the volume of the object. 17 1. Obtain an unknown irregularly shaped object from your instructor and record the number. 2. Weigh the object and record the mass in grams. 3. Fill a 1000 ml graduated cylinder to about the 700 ml mark with water. Record the exact volume. 4. Carefully submerge the object in the water so that no water is lost. 5. Record the new volume of the water. 6. The difference between the initial volume of water and the final volume of water is the volume in ml of the object. 7. Calculate the density in g/ml of the object. PART D - DENSITY OF A REGULARLY SHAPED OBJECT Measuring the volume with a metric ruler and weighing the object can determine the density of a regularly shaped object. 1. Obtain a cube, triangle, and cylinder from your instructor. Record the unknown numbers. 2. Weigh each object and record the mass in grams. 3. Measure the objects with the metric ruler and perform the calculations as in the examples in the discussion section. 4. Calculate the density of each object. 18 NAME ________________ DATE _________________ SECTION _____________ MEASUREMENTS REPORT SHEET EXPERIMENT 2 ACCURACY IN LIQUID MEASUREMENTS 1. ml deviation between beaker and cylinder = (ml beaker - ml cylinder) _______________ 2. ml deviation between flask and cylinder = (ml flask - ml cylinder) _______________ 3. volume of test tube _______________ 4. volume of gas bottle _______________ What does the differences between the graduated cylinder and the flask and beaker indicate? Water THE DENSITY OF A LIQUID Unknown No. __ 1. Mass of cylinder and liquid (g) _________________ __________________ 2. Mass of empty cylinder (g) _________________ __________________ 3. Mass of liquid (g) _________________ __________________ 4. Volume of liquid (ml) _________________ __________________ 5. Density of liquid (g/ml) _________________ __________________ What is the density of the unknown liquid in g/cm3? 19 DENSITY OF AN IRREGULARLY SHAPED OBJECT Unknown No._____ 1. Mass of object (g) ________________ 2. Initial volume of liquid (ml) ________________ 3. Volume of liquid after immersion (ml) ________________ 4. Volume of object (ml) ________________ What is the density (g/ml) of the object? DENSITY OF REGULARLY SHAPED OBJECTS Cube Triangle Cylinder 1. Unknown number ___________ ___________ ___________ 2. Height (cm) ___________ ___________ ___________ 3. Width (cm) ___________ ___________ XXXXXXXX 4. Thickness (cm) ___________ ___________ XXXXXXXX 5. Diameter (cm) XXXXXXXX XXXXXXXX ___________ 6. Radius (cm) XXXXXXXX XXXXXXXX ___________ 7. Volume (cm3) ___________ ____________ ___________ 8. Mass (g) ___________ ____________ ___________ 9. Density (g/cm3) ___________ ____________ ___________ 20