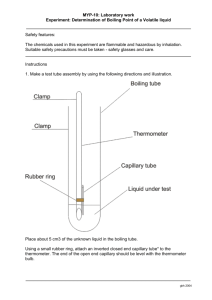
Boiling Point Laboratory 1. Purpose: To determine the effects on
advertisement

Boiling Point Laboratory 1. Purpose: To determine the effects on boiling point when two liquids, alcohol and water, are mixed together. 2. Hypothesis: When two liquids are mixed, will they (1) retain their individual characteristics, i.e. boiling point? (2) Will the amount of the mixture effect the boiling point? (1)_______________________________________________________________________ __________________________________________________________________________ (2)_______________________________________________________________________ __________________________________________________________________________ 3. Safety Box: (1) eyewear, (2) burner safety, (3) glass/rubber safety, (4) chemicals are flammable and also toxic if ingested; wash hands if contact, (5) proper chemical handling, (6) thermometer care, (7) boiling chips, (8) tubing position + removal, (9) use the burner insert to keep the liquid barely boiling – start with the test tube đ on and đ off the white edge. (10) Remove tubing from test tube and place behind pegboard BEFORE turning burner off. Failure to do so may result in a backflush or explosion. 4. Centigrade thermometer known and guess values. Readability to the đo Celsius; every reading must be recorded as .0 or .5oC. 5. AMOUNTS: Stations 1-6 uses ~5mL, 7-12 uses ~10mL 6. TAKE temperature every 30 seconds until ALMOST DRY. If you have had the same temperature for 5 minutes and are almost dry, you may stop 7. Create a single line graph, plotting and connecting the data points from the thermometer. Temperature values are 2o per box on the “Y” (tall) axis, from 100o+ to 20oC (40 boxes). Time in on the “X” axis, starting at “0”, with each box at 30 seconds (about 30 boxes). Directions 1. Set up station as seen in the demo station. Water bath is đ full, using the 600mL beaker. 2. Add a touch of glycerin to the stopper and rubber tubing using the wooden splint. Too much and the stopper will not stay in place. 3. Add 3-4 boiling chips to the test tube. Using a paper towel, grasp the class tubing and gently slide the rubber tubing onto the glass about 1cm, or a fingernail’s distance. 4. Put the stopper into the test tube; loop the tubing around the test tube tongs and into the test tube about 1cm off the bottom. During the lab as liquid collects you may need to move the tubing up to maintain this distance. 5. Record the T0 of the experiment. 6. Light the burner and place the flame so that it is directly under the test tube, on the edge of the ceramic circle of the burner cover. Using tongs (not rubber beaker tongs) or tweezers, move the ceramic circle to speed up or slow down the boiling so as to finish in about 10 minutes. Do not adjust until liquid has begun to boil. 7. When almost dry, ~10 minutes, remove the tubing FIRST and place behind the pegboard – then turn the burner off. . End is amount dependent, not time dependent. 8. When cool, remove the stopper. Using test tube tongs, carry the test tube to the front and pour into the large flask, boiling chips and all. Also pour the small test tube into the flask. 9. Rinse both test tubes into the sink and dump out the water bath. 10. Remove the rubber tubing in the same manner as out put it on. Call for help if stuck – do not force it. Place the thermometer upside down, hanging on the top of the pegboard with the tubing draped around it. 11. Spray down the counter and wipe with a damp sponge. 12. Start your graph. Boiling Point Data Chart All data must be recorded to the .0 or .5oC Thermometer error: +/- 1oC Completion time (almost dry – liquid down to the boiling chips) should be about 10 minutes; not to exceed 15 minutes. Time (m) Temperature (oC) Time (m) 0 8.0 0.5 8.5 1.0 9.0 1.5 9.5 2.0 10.0 2.5 10.5 3.0 11.0 3.5 11.5 4.0 12.0 4.5 12.5 5.0 13.0 5.5 13.5 6.0 14.0 6.5 14.5 7.0 15.0 7.5 Temperature (oC) Conclusion: Address both parts of the hypothesis ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Questions 1. What happened on the graph; are there one or two boiling points? How do you know? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. What happens to the temperature when each of the liquids begins to boil; why? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 3. What is happening to the water molecules when the alcohol is boiling? What is the role of the Heat of Vaporization? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 4. Did the sample size affect the boiling point? See the data from the other side of the room. Is boiling point an intensive or extensive physical property? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________