Lab 3.11 Boiling Point
advertisement

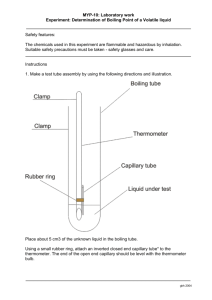
Name _______________________________________________________ Period ________________ Lab Partners: __________________________________________________Date submitted___________________ Lab 3.11 Boiling Point Purpose: Use boiling point as a characteristic property to identify an unknown liquid. Hypothesis: ____________________________________________________________________________________ ____________________________________________________________________________________ Rationale: ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ Materials: Safety glasses Pegboard Fire stand and burner Large test tube Small test tube Two holed rubber stopper with tubing and thermometer Boiling Chips Stop watch 2 Clamps Beaker (250ml) Safety: Safety goggles, proper handling of chemicals and glassware Draw a labeled diagram of the apparatus Procedure: (see page 59 in text) 1. Set up apparatus as shown in Figure 3.7 on page 59. Make sure the thermometer touches water only and not glass, and that it is completely submerged. Add 1-2 boiling chips to prevent uneven boiling. 2. Adjust the thermometer in the test tube so that you can read it at the cork’s slit. 3. Heat the liquid in the test tube and record the temperature every half-minute until the liquid has been boiling for about 5 minutes. 4. On your data table, highlight or mark with an asterisk the temperature of the liquid just as it begins to boil. 5. Plot a graph of the temperature of the liquid as a function of time. Lab Group Data TIME MIN C SUBS. Class Boiling Point Data TIME MIN C SUBS. TIME MIN 0 10 20 0.5 10.5 20.5 1 11 21 1.5 11.5 21.5 2 12 22 2.5 12.5 22.5 3 13 23 3.5 13.5 23.5 4 14 24 4.5 14.5 24.5 5 15 25 5.5 15.5 25.5 6 16 26 6.5 16.5 26.5 7 17 27 7.5 17.5 27.5 8 18 28 8.5 18.5 28.5 9 19 29 9.5 19.5 29.5 C SUBS. Substance A (C) Vol (mL) Substance B (C) Sample________ Sample Volume __________ Boiling Point __________ Vol (mL) Do all the graphs look alike at the beginning of the curve? ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ Do all the graphs have a flat section? ____________________________________________________________________________________ ____________________________________________________________________________________ Did the other teams with the same liquid get the same boiling temperature? ___________________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ___________________________________________________________________________________ What does a difference in boiling point reveal? ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ Does the boiling point of a liquid depend on the amount of liquid? ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ Conclusion: Is boiling point a characteristic property? Use evidence to explain your answer. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ _____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________