Chapter (1 Part 1) Page 1 of 8
advertisement
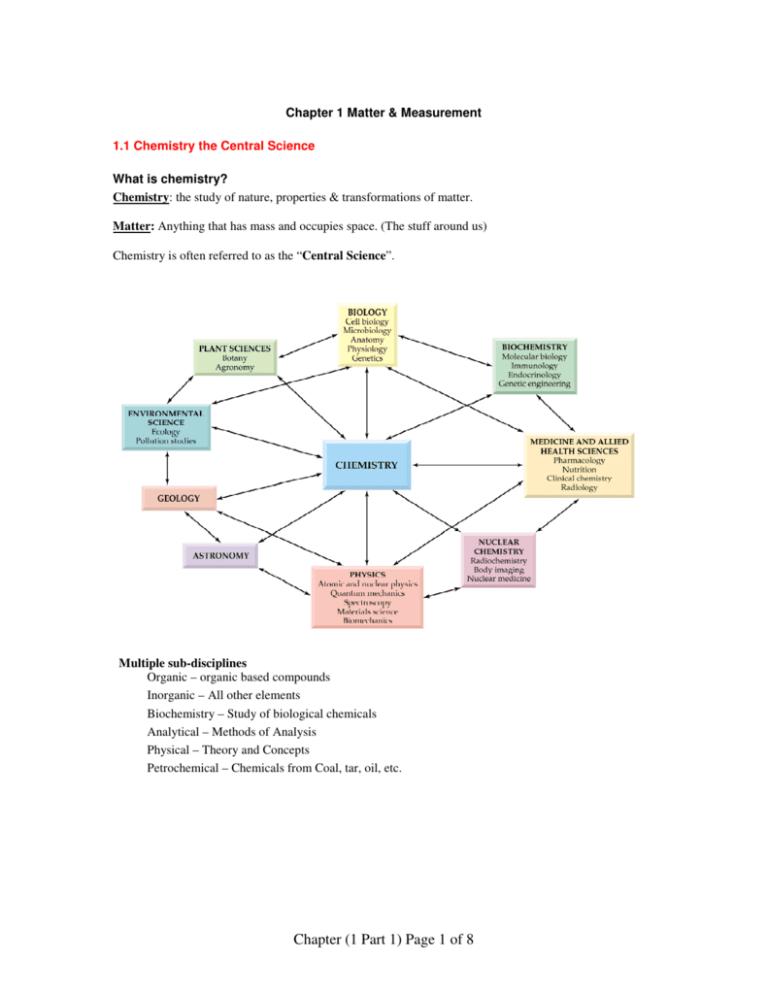
Chapter 1 Matter & Measurement 1.1 Chemistry the Central Science What is chemistry? Chemistry: the study of nature, properties & transformations of matter. Matter: Anything that has mass and occupies space. (The stuff around us) Chemistry is often referred to as the “Central Science”. Multiple sub-disciplines Organic – organic based compounds Inorganic – All other elements Biochemistry – Study of biological chemicals Analytical – Methods of Analysis Physical – Theory and Concepts Petrochemical – Chemicals from Coal, tar, oil, etc. Chapter (1 Part 1) Page 1 of 8 Chemical and Physical Properties Physical Property A characteristic that can be observed without changing the composition of the substance. (e.g., size, color, weight) Qualitative Quantitative Color Melting point Odor Boiling point Taste Weight Chemical Property Describes how a substance reacts with other substances. These result in a change in the composition of a material as a result of a chemical reaction. Problem: Are the following examples of chemical or physical properties? type? Lead is denser than aluminum _____________ oxygen gas supports combustion _____________ Water boils below 100 degrees at altitudes _____________ Physical and Chemical Changes Physical Changes DO NOT alter the chemical makeup of a substance. e.g., Phase changes are examples of physical changes. Chemical Changes DO alter the chemical makeup. They involve a chemical reaction e.g., a nail rusting or burning the fuel in your car. Problem: Are the following examples of chemical or physical changes? Type of Change? Milk turning sour making wine from grapes water freezing plants undergoing photosynthesis helium leaking out of a balloon _________ _________ _________ _________ _________ Chapter (1 Part 1) Page 2 of 8 1.2 States of Matter States of Matter − Matter exists in three phases; Phase depends on temperature and pressure Changes of State _________ to go from a liquid to a solid _________ to go from a solid to a liquid _________ to go from a liquid to a gas _________ to go from a gas to a liquid _________ to go directly from a solid to a gas (without first becoming a liquid) _________ to go from a gas directly to a solid Problem: Acetic acid, (which gives the sour taste to vinegar), has a melting point of 16.7 °C and a boiling point of 118 °C. Predict the physical state of acetic acid when the ambient temperature is 10 °C. . solid 10 °C m.p. 16.7 °C liquid b.p. 118 °C (End of Day 1) Chapter (1 Part 1) Page 3 of 8 Gas 1.3 Classification of Matter Example of a chemical process that would break up a compound into its elements: Emphasize reactants, products and arrow. Problem: Classify each of the following as a mixture or a pure substance. If a mixture – decide homogeneous or heterogeneous. If a pure substance – decide compound or element. a) Vanilla ice cream – Mixture: Homogenous b) Sugar – Pure Substance: Compound Problem: how many elements of each type are in one formula unit of Al2(S2O3)3? Chapter (1 Part 1) Page 4 of 8 1.5 Elements and the Periodic Table Periodic Table • Developed by Demitri Mendeleev - Hand out Periodic Tables • Arranged so that elements with similar properties are grouped together. (see next section) • Metals (94 of 118): (to the left of the stair-step line) • Semimetals or Metalloids have properties between that of a metal and a nonmetal. semimetals: Textbooks differ, semimetals are Si, Ge, As, Sb, Te, (Sometimes boron (B), astatine(At) and/or polonium (Po). We will consider all elements touching the line (except Al) as a semimetal. • Nonmetals: (to the right of the stair-step line) (Metals to the left of the line, nonmetals to the right) Chapter (1 Part 1) Page 5 of 8 Elements to Know Aluminum Al 13 Manganese Mn 25 Antimony Sb 51 Mercury Hg 80 Argon Ar 18 Molybdenum Mo 42 Arsenic As 33 Neon Ne 10 Barium Ba 56 Nickel Ni 28 Beryllium Be 4 Nitrogen N 7 Bismuth Bi 83 Oxygen O 8 Boron B 5 Phosphorus P 15 Bromine Br 35 Plutonium Pu 94 Cadmium Cd 48 Potassium K 19 Calcium Ca 20 Radium Ra 88 Carbon C 6 Radon Rn 86 Cesium Cs 55 Rubidium Rb 37 Chlorine Cl 17 Selenium, Se 34 Chromium Cr 24 Silicon Si 14 Cobalt Co 27 Silver Ag 47 Copper Cu 29 Sodium Na 11 Fluorine F 9 Strontium Sr 38 Gold Au 87 Sulfur S 16 Helium He 2 Thallium Tl 81 Hydrogen H 1 Tin Sn 50 Iodine I 53 Titanium Ti 22 Iron Fe 26 Tungsten W 74 Krypton Kr 36 Uranium U 92 Lead Pb 82 Vanadium V 23 Lithium Li 3 Xenon Xe 54 Magnesium Mg 12 Zinc Zn 30 Chapter (1 Part 1) Page 6 of 8 Metallic Character Metal State Appearance Pliability Conductivity Density Melting pt. Reactivity Solid (liq Hg) Shiny when cut Malleable (bendable) ductile (draws into wire) Good conductor Usually high Usually high With nonmetals Nonmetal Solid, gas(liq Br2) Dull Brittle Poor cond Usually low Usually low With metals or nonmetals 1.6 Chemical Reactions: An example of Chemical Change. Reactions The process of ethanol burning in oxygen is an example of a chemical reaction. We can write a chemical equation to show what is happening in the reaction: CH3CH2OH + 3O2 2 CO2 + 3 H2O Reactants are elements and compounds on the left of arrow. Products are elements and compounds on the right of arrow. Evidence of Chemical Reaction: 1. Precipitate form 2. Gas formation 3. Color formation 4. Heat Chapter (1 Part 1) Page 7 of 8 Matter Mixtures homogeneous Pure Substances heterogenous elements monotomic metalic properties metals semimetals same atoms molecules diatomics and higher nonmetals Chapter (1 Part 1) Page 8 of 8 different atoms covalent compounds ionic