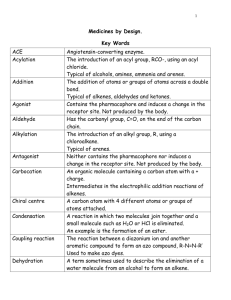
Pharmacophores Revisited
advertisement

DavidsonX – D001x – Medicinal Chemistry Chapter 9 – Binding, Structure, and Diversity Part 3 – Drug-Target Complementarity Video Clip – Pharmacophores Revisited Intermolecular forces hold a drug to its target. The intermolecular forces will not form, however, unless the drug's functional groups are oriented to interact strongly the the functional groups in the target. In other words, a drug needs the right functional groups with the correct geometry in order to bind strongly to a given target. Proper functionality and geometry describe a drug's pharmacophore. The idea of pharmacophores were introduced back in Chapter 1. The pharmacophore of morphine and the sulfa drugs were discussed in terms of very molecular scaffolds. (1) benzene ring (3) two carbon linker morphine pharmacophore O O S R N H H2N N (4) tertiary amine (2) quaternary carbon sulfonamide antibiotic pharmacophore R = H, acyl, aryl, heteroaryl Pharmacophores are more often described in terms of functional groups and their ideal spacing. A hypothetical example is shown below. The pharmacophore consists of a hydrogen bond donor, a negatively charged group, and a large non-polar group each separated by a certain distance. non-polar group hydrogen bond donor negatively charged group To satisfy these functional groups, one might select an alcohol (hydrogen bond donor), carboxylate (negatively charged group), and a phenyl ring (non-polar group). Furthermore, these might be connected as shown in compound 1 to give the appropriate spacing. H alcohol O H O phenyl O O carboxylate O molecule satisfying pharmacophore 1 O A problem with structure 1 is its conformational flexibility. While the structure is drawn to show an ideal orientation of the key functional groups, the molecule has a huge number of conformations that would improperly space the functionality. Indeed, some medicinal chemists recommend downgrading hits that have a high number of freely rotating bonds. The most common method for restricting flexibility of a molecule is the incorporation of rings or double bonds. Several possible restricted structures are shown below. Compounds 2 through 4 incorporate alkenes. Alkenes restrict conformations somewhat. Compounds 4 through 7 incorporate rings at different positions. Rings can greatly restrict flexibility and hold functional groups in a desired relative orientation. A problem with introducing rings is that they normally add extra carbons to the molecule. New carbon might add steric bulk to the structure and prevent binding. H O H O 2 H O 4 O O 6 O O H O O H O H O 3 7 5 O O O O O O O Pharmacophores often include many key functional groups, certainly more than just three. With four functional groups, unless they happen to lie within a plane, the pharmacophore will occupy a three-dimensional space. With three-dimensional space comes the importance of stereochemistry. While many drugs do not contain stereocenters, some do. Those that do contain stereocenters are often approved exclusively in a single enantiomer form. In these cases, the correct stereochemical configuration is normally required to achieve proper threedimensional orientation of the drugs functional groups for proper activity. Two examples of drugs that are marketed as single enantiomers are naproxen (8) and duloxetine (9). CH3 S CO2H O CH3O naproxen (Aleve) analgesic 8 duloxetine (Cymbalta) antidepressant N H CH3 9