For each structure below:
advertisement
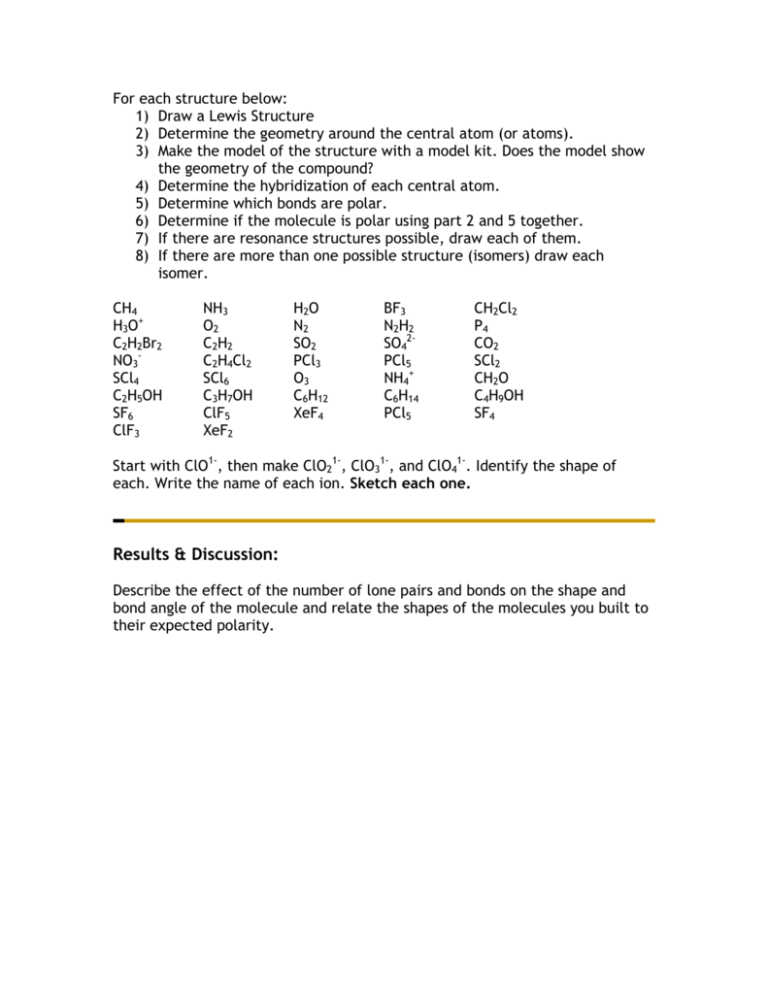
For each structure below: 1) Draw a Lewis Structure 2) Determine the geometry around the central atom (or atoms). 3) Make the model of the structure with a model kit. Does the model show the geometry of the compound? 4) Determine the hybridization of each central atom. 5) Determine which bonds are polar. 6) Determine if the molecule is polar using part 2 and 5 together. 7) If there are resonance structures possible, draw each of them. 8) If there are more than one possible structure (isomers) draw each isomer. CH4 H3 O+ C2H2Br2 NO3SCl4 C2H5OH SF6 ClF3 NH3 O2 C 2 H2 C2H4Cl2 SCl6 C3H7OH ClF5 XeF2 H2O N2 SO2 PCl3 O3 C6H12 XeF4 BF3 N 2 H2 SO42PCl5 NH4+ C6H14 PCl5 CH2Cl2 P4 CO2 SCl2 CH2O C4H9OH SF4 Start with ClO1-, then make ClO21-, ClO31-, and ClO41-. Identify the shape of each. Write the name of each ion. Sketch each one. Results & Discussion: Describe the effect of the number of lone pairs and bonds on the shape and bond angle of the molecule and relate the shapes of the molecules you built to their expected polarity.