CHMA – Lewis Dot Structures of Molecules © Van Der Sluys, 2004
advertisement

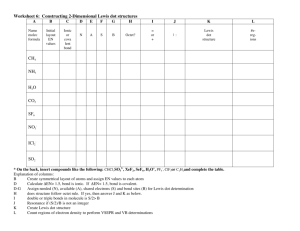
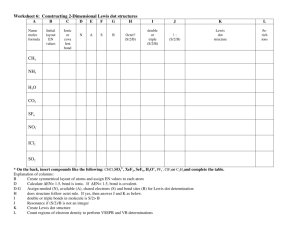
Name _________________________ CHMA – Lewis Dot Structures of Molecules In order to understand the covalent bonding in a molecule or polyatomic ion, it is often useful to draw a Lewis structure of the molecule. In a Lewis structure only the valence electrons are shown. For anions you will need to add one additional electron for each negative charge. This electron should usually be given to the most electronegative atom in the formula. For cations, you will need to remove one electron for each positive charge. Usually remove the electrons from the least electronegative atom. Draw Lewis dot structures for the following. 1. HCl 2. H2O 3. Cl2O 4. NH3 5. N2 6. O2 7. HCN 8. CH4 9. C2H2 10. C2H4 11. PCl3 12. C2H6 13. BH4- 14. OF2 © Van Der Sluys, 2004 Name _________________________ CHMA – Lewis Dot Structures of Molecules 15. BF3 16. NH4+ 17. CCl4 18. CH3OH 19. H2S 20. ClO- 21. CCl2F2 22. CO2 23. C3H8 24. CH3F 25. CH3CH2OH 26. CH3OCH3 © Van Der Sluys, 2004 Name _________________________ CHMA – Lewis Dot Structures of Molecules Answers .. 1. H-Cl: .. 2. .. .. 3. :Cl-O-Cl: .. .. .. H-O-H .. .. 4. H-N-H H .. .. 6. :O O: 5. :N N: H 8. H-C-H H 7. H-C N: 9. H-C C-H 10. H-C C-H H H .. .. .. 11. :Cl-P-Cl: .. .. :Cl: .. H H 12. H-C C-H H H H 13. H-B-H H .. .. 15. :F-B-F: .. .. :F: .. 17. - .. .. .. 14. :F-O-F: .. .. .. H .. :Cl: .. .. :Cl-C-Cl: .. .. :Cl: .. + 16. H-N-H H 18. .. H-C-O-H .. H .. 19. H-S-H .. .. :F: .. .. 21. :Cl-C-Cl: .. .. :F: .. HHH 23. H-C-C-C-H HHH .. .. 20. [:Cl-O:] .. .. H H .. 25. H-C-C-O-H .. HH H .. H 26. H-C-O-C-H .. H H © Van Der Sluys, 2004 H .. .. 22. :O=C=O: .. :F: 24. H-C-H H