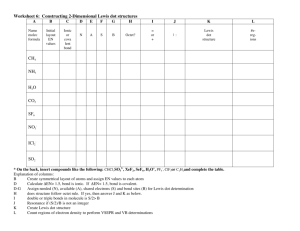
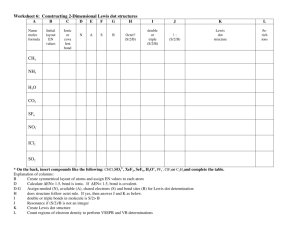
Chemical Bonds, Lewis Dot Structures & Formal Charge Worksheet

Name _________________________________________ Pd ____ Date ______________________
Chemistry: Chemical Bonds, Lewis Dot Structures & Formal Charge Worksheet
Watch the video lesson on Formal Charges before completing the worksheet. The video lesson is a graded assignment (the worksheet is not a graded assignment). You may go back through the video lesson a second time and answer the questions you got wrong for partial credit.
Intro to Formal Charge
Formal charge is a useful concept that when used with Lewis dot structures eliminate many of the shortcomings of the octet rule/Lewis dot structure model. Namely, the formal charge/Lewis dot structure model can predict
when central atoms will have incomplete or expanded octets
the correct structure when the octet rule predicts there is more than one valid structure
1.
How is formal charge used to predict when a central atom will have an incomplete or expanded octet?
2.
How is formal charge used to predict the correct structure from several Lewis dot structures that the octet rule deems valid?
Lewis Dot Structures
3.
Determine the total number of valence electrons and formal charges in each of the following covalent compounds or polyatomic ions. Then draw the Lewis dot structure showing the valence electrons and formal charges for each atom. a.
SBr
2 b.
NBr
3
c.
NH
3 d.
SO
3
2f.
H
3
O
+ g.
SiF
4 e.
NO
2
2h.
C
2
H
6
4.
When does a covalent compound have resonance structures?
5.
Draw the Lewis dot structure for each of the following molecules or polyatomic ions and indicate the formal charge: a.
HCCH b.
CO
c.
H
2
CO (C is the central atom) g.
O
2
2d.
SF
4 e.
N
2
O (N N O) f.
F
2 j.
SF
6 h.
XeF
2 i.
PI
5
k.
SbCl
5 l.
XeF
4
6.
Draw the Lewis dot structure for each of the following ionic compounds: a.
MgO d.
Li
2
O b.
BaF
2 c.
AlCl
3 e.
Al f.
K
3
2
O
N
3