Evaporation and
advertisement

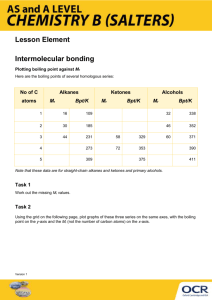
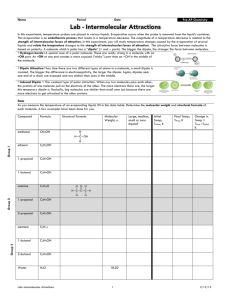
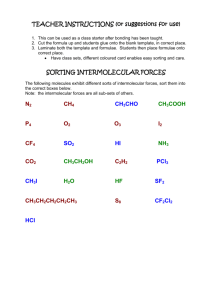
Academic Chemistry Lab: Intermolecular Force Study (34 points) (page 1 of 3) Reference: Zumdahl Chapter 10 section 1 Intermolecular Forces Zumdahl Chapter 22 section 1 Alkanes Zumdahl Chapter 22 section 4 Hydrocarbon Derivatives Background: In this investigation, temperature probes are placed in various liquids. Evaporation occurs when the probe is removed from the liquid’s container. This evaporation is an endothermic process that results in a temperature decrease. The magnitude of a temperature decrease is, like viscosity and boiling temperature, related to the strength of intermolecular forces of attraction. In this investigation, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. You will encounter two types of organic compounds in this investigation—alkanes and alcohols. The two alkanes are pentane, C5H12, and hexane, C6H14. In addition to carbon and hydrogen atoms, alcohols also contain the -OH functional group. Methanol, CH3OH, and ethanol, C2H5OH, are two of the alcohols that we will use in this investigation. You will examine the molecular structure of alkanes and alcohols for the presence and relative strength of two intermolecular forces—hydrogen bonding and dispersion forces. Materials: IBM compatible computer LabPro Logger Pro two temperature probes six 3cmX3cm filter paper rubber bands, or Al wire iron ring and stand methanol (CH3OH): flamiable and poisionous ethanol (C2H5OH): denatured, flammiable and poisionous 1-propanol (C3H7OH): flamiable and poisionous 1-butanol (C4H9OH): flammable and poisonous n-pentane (C5H12): flammable and poisonous n-hexane (C6H14): flammable and poisonous Pre-lab exercise: Prior to doing this investigation, complete the Pre-Lab table. The name and formula are given for each compound. Draw a structural formula for a molecule of each compound. Then determine the molar mass of each of the molecules. London intermolecular forces exist between any two molecules, and the attractive force increases as the molar mass of the molecule increases. Next, examine each molecule for the presence of hydrogen bonding. Before hydrogen bonding can occur, a hydrogen atom must be bonded directly to an N, O, or F atom within the molecule. Tell whether or not each molecule has hydrogenbonding capability. Academic Chemistry Lab: Intermolecular Force Study (34 points) (page 2 of 3) Procedure: 1. Plug Temperature Probe 1 into Channel 1 and Temperature Probe 2 into Channel 2 of the LabPro interface. Connect the computer to the LabPro using the interface cable and boot LoggerPro 2. To up the data collection time select expirment/data-collection. The mode should be time based. Collect data for about 240seconds (4minutes). There should be about one reading per second. 3. Wrap Probe 1 and Probe 2 with a 3cm by 3cm square pieces of filter paper and secure with a small rubber band. You are trying to manke a paper skirt and not a paper sock. The paper should be even with the probe end. Suspend these probes from an iron ring so that they hang freely. 4. Find your goggles. Pour about 1cm of ethanol and 1cm 1-propanol into two separate test tubes. Insert probe 1 in the ethanol and probe 2 in the 1-propanol. Take special care not to confuse the two. 5. After the probes have been in the liquids for at least 30 seconds, tap to begin collecting temperature data. A live graph of temperature vs. time for both Probe 1 and Probe 2 is being plotted on the handheld screen. The live readings are displayed in the lower left corner. Monitor the temperature for 15 seconds to establish the initial temperature of each liquid. Then simultaneously remove the probes from the liquids. 6. Data collection will stop after 4 minutes (or tap if you want to end data collection before 4minutes has elapsed). 7. If you are satified with your graph scroll through the data table and record the maximum temperature, and minimum temperature for each chemical 8. Adjust your scale so all data is graphed. Title you graph and call out which chemical is on which temperature probe. Remember the printer only does black and white. 9. Repeat Steps 4-8 using 1-butanol, n-pentane, methanol, and n-hexane. Use new filter paper for each chemical studied. Calculations: 1. Calculate t for each chemical studied. 2. Plot t versus molar mass using data from the four alcohols. Put molar mass on the horizontal axis and t on the vertical axis. If the data is good a smooth relationship should be seen here. 3. Select any best fit curve that produces a low root mean square error (RMSE). Name: ________________________ period: _______ (34 points) (page 3 of 3) Academic Chemistry Computer Lab: Intermolecular Force Study Pre-lab data table: (2 points) Substance Formula methanol (an alcohol) CH3OH ethanol (an alcohol) C2H5OH 1-propanol (an alcohol) C3H7OH 1-butanol (an alcohol) C4H9OH n-pentane C5H12 n-hexane C6H14 Structural Formulas Molar Mass (g/mole) Hydrogen Bond (Yes or No) Data table: (2 points) Substance tmax (°C) tmin (°C) t (tmax–tmin) (°C) methanol (an alcohol) ethanol (an alcohol) 1-propanol (an alcohol) 1-butanol (an alcohol) n-pentane n-hexane Questions: (2 point each) 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molar mass, but significantly different t values. Explain the difference in t values at the atomic level. 2. Which of the alcohols studied has the strongest intermolecular forces of attraction? ____________The weakest intermolecular forces? ________ 3. Explain your answer for question two at the atomic level. 4. Which of the alkanes studied has the stronger intermolecular forces of attraction? ____________ Which has the weaker intermolecular forces? ___________ 5. Explain your answer for question 4 at the atomic level. Note: Each temp v/s time graph for the six chemicals studied. (12 points total). The alcohol temperature change v/s molar mass graph is 8 points. Page 3 of 3 completed. (14 points) Remember: No credit for xerox or traced copies.