File
advertisement

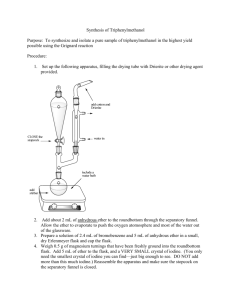
Synthesis of a Carboxylic Acid from an Unknown Alkyl Bromide Beau Duty* Department of Chemistry, Harding University, Box 12272, Searcy, AR 72149. Bduty@harding.edu KEYWORDS. Synthesis; Carboxylic Acid; Alkyl Halide; Grignard Reaction; Reflux and Extraction. INTRODUCTION. The Nobel Prize of 1912 was awarded to Victor Grignard for the development of organomagnesium halide complexes (Grignard Reagents) which he used in the synthesis of alcohols1. The Grignard reaction is an effective method of forming new carbon to carbon bonds. This specific variation of the Grignard is one of the more facile ways of adding a carboxylic acid to a molecule. One of the more interesting aspects of the reaction is that it must be conducted in an ether solvent because the ether is crucial to the formation of the Grignard reagent, which takes on a conformation similar to that shown below. This is the first step of the reaction. H3C [Mg] H3C CH3 O O Br CH3 Figure 1. Grignard Reagent. 1 The reaction then proceeds as the carbon at which the halide was previously attached acts as a nucleophile and attacks the carbon in carbon dioxide to form a benzoate ion. See figure 2. O O [Mg] Br - O + O Figure 2. Formation of the Benzoate Ion. Subsequent washing of the mixture with water protonates the anionic oxygen and yields our carboxylic acid as seen in figure 3. O O H - O + OH O H Figure 3. Protonation of Anionic Oxygen. Include a theoretical yield as needed. The goal of the experiment is to synthesize a carboxylic acid from an unknown alkyl halide, and to use the product to identify the unknown alkyl halide. The formation of the Grignard reagent will be conducted under reflux. The reagent will then be poured over crushed dry ice to synthesize the acetate intermediate, and subsequent washing of the mixture with water will yield the carboxylic acid product. Characterization of the product using Infrared 2 Spectroscopy (IR) and Nuclear Magnetic Resonance (NMR), as well as a melting point will allow for identification of the unknown alkyl halide reagent used in the synthesis. EXPERIMENTAL METHODS. All glassware will be cleaned and thoroughly dried well before lab begins to ensure no water is present in any part of the reaction assembly. The pieces will be stored in the oven until the time of their use has come, then they will be removed and rapidly assembled. A drying tube will be stopped with glass wool and filled with anhydrous calcium chloride, then fitted to the assembly. An ice bath will be prepared to cool the reaction if it becomes too violent. One gram of magnesium turnings and a stir bar will be added to the round-bottomed flask. A separatory funnel will then be used to add 2-3mL of dry diethyl ether into the reaction assembly. The reaction flask will be heated until fumes from the ether are wafting from the drying tube. The setup will look similar to the diagram below. Drying Tube Water out West Condenser Separatory Funnel Water In Claisen Adapter 100 mL Round Bottom Flask Figure ?. Diagram of Reaction Assembly. The separatory funnel will then be used to add 4-5mL of dry alkylbromide and 8-10mL of dry diethyl ether; the flask will be swirled to mix the chemicals. The water will be started in the condenser, and the magnetic stirrer will be started. 2-3mL of the mixture in the separatory funnel will be added to the 3 reaction flask. Then the flask will be heated by cupping it in the hands until a cloudiness is present in the flask, which indicates the exothermic reaction has started. The cloudiness will be proceeded by bubbles, and finally a color change. The reaction will be watched with care so it does not get out of control. As the reaction proceeds, the remaining mixture in the separatory funnel will be added to the reaction mixture until all has been reacted. If the reaction does not immediately begin it will be spurred in one of three ways; by using a heating mantle; by seeding with iodine crystals; or by using a starter. 23mL of diethyl ether will be added to the separatory funnel, and subsequently to the reaction flask to rinse any remaining alkyl halide from the separatory funnel. The reflux will be run for approximately 20 minutes after the addition of all the alkyl bromide, most of the magnesium should be gone. The reaction flask will be left to cool to room temperature, and then dry diethyl ether will be added until the cumulative volume is about 40mL. Five grams of crushed dry ice will be obtained and the reaction mixture will be poured slowly into the beaker of dry ice, and the resulting mixture will be stirred vigorously. Once all of the ice has sublimed, the product (usually a brown, highly viscous mass) will be acidified with 3M HCl. If any solid remains it will be dissolved with added diethyl ether. The aqueous phase will be extracted two or more times and the ethereal layers will be combined. The ethereal layers will be extracted using 15mL of 1.25M NaOH a minimum of two times. The combined aqueous solutions will be acidified with 6M HCl until acidic to litmus. The solution will be cooled in an ice bath to complete precipitation. The solid will be collected using vacuum filtration and the crystals will be dried using first, a porous plate, and then the drying oven. The mass of the carboxylic acid will be taken and a percent yield determined. The product will be identified using melting point, IR, and 1H NMR. Table 1 – Chemicals to be used in the synthesis of a carboxylic acid. Chemical Molecula r Weight Boiling Melting Point (0C) Point (0C) Density HMIS Labels Safet Other: y Note 4 (amu) Benzoic Acid Bromobenze ne Calcium Chloride Diethyl Ether Hydrochlori c Acid Magnesium Sodium Hydroxide REFERENCES. Place your list of references at the end of the manuscript. References should be cited sequentially by superscript numbers at the end of the appropriate sentence. Authors should check all parts of each reference listing against the original document. Each reference should be written in J. Am. Chem. Soc format. You cannot have too many references. The general rule is that anything not common knowledge must be referenced. Only references from primary sources are allowed. 5 (1) Grutzner, J. B.; Stewart, K. W.; Wasylishen, R. E.; Lumsden, M. D.; Dybowski, C.; Beckmann, P. J. Am. Chem. Soc. 2001, 123, 7094-7100. (2) Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961; Chapters VIII and IX. 6