Cabbage Juice Titration Lab
Introduction: Red cabbage juice is an example of an acid-base
indicator. In chemistry, an indicator is used to detect the presence of a
specific chemical or a specific type of chemical. In this lab, red
cabbage juice is used to indicate whether a solution is acidic or basic.
Red cabbage contains the pigments flavonol and anthocyanin. The
anthocyanin pigment causes very strong acids to appear red. It causes
neutral or weak basic solutions to appear blue, and strong basic
solutions to appear colorless. Flavonol, on the other hand, causes
strong acidic solutions to appear colorless and strong basic solutions to
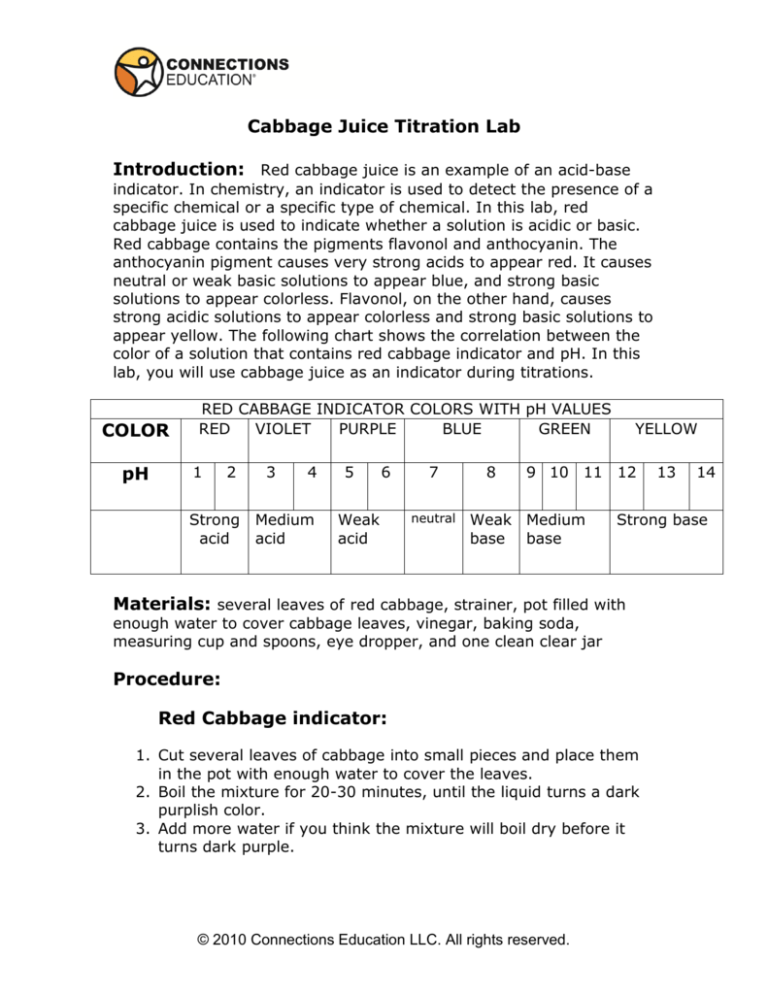
appear yellow. The following chart shows the correlation between the
color of a solution that contains red cabbage indicator and pH. In this
lab, you will use cabbage juice as an indicator during titrations.
COLOR
pH
RED CABBAGE INDICATOR COLORS WITH pH VALUES
RED
VIOLET
PURPLE
BLUE
GREEN
1
2
Strong
acid
3
4
Medium
acid
5
6
Weak
acid
7
neutral
8
YELLOW
9 10 11 12
Weak Medium
base base
14
Strong base
Materials: several leaves of red cabbage, strainer, pot filled with
enough water to cover cabbage leaves, vinegar, baking soda,
measuring cup and spoons, eye dropper, and one clean clear jar
Procedure:
Red Cabbage indicator:
1. Cut several leaves of cabbage into small pieces and place them
in the pot with enough water to cover the leaves.
2. Boil the mixture for 20-30 minutes, until the liquid turns a dark
purplish color.
3. Add more water if you think the mixture will boil dry before it
turns dark purple.
© 2010 Connections Education LLC. All rights reserved.
13
4. Transfer the fluid into a glass or jar, pouring it through a strainer
to remove the cabbage pieces.
Using cabbage juice as an indicator during titration:
1.
2.
First you need to calibrate your eyedropper.
Figure out how many drops of water are in 1/8 of a teaspoon.
Multiply that number by 8 to obtain the number of drops per
teaspoon.
# of drops/tsp = ________________________
3.
1 teaspoon is equal to 4.93 mL. Divide the number of drops per
teaspoon by 4.93 to obtain the number of drops per ml. You will
use this ratio to convert number of drops to mL when you
perform your calculations.
# of drops/mL = _______________________
4.
Next, combine 1 teaspoon of vinegar with ½ tablespoon of red
cabbage indicator in a small container and set it aside. This
solution is to be used for comparison when you perform your
titration.
5. Combine 1 teaspoon of water with ½ tablespoon of red cabbage
indicator in a separate container and set it aside. This solution
will also to be used for comparison when you perform your
titration.
6. Next, mix 4¾ level teaspoons (28.35 g or 1 oz) of baking soda
with 1¼ cup (295.74 ml) of water in a small container and set it
aside. This will give you a solution with a concentration of 1.14
M.
7. Transfer 2 teaspoons (9.86 ml) of vinegar into a clean clear jar.
8. Add 1 tablespoon of cabbage juice to the clear jar that contains
the vinegar.
9. Use the eye dropper to add the sodium bicarbonate to the
vinegar one drop at a time. Keep track of the number of drops
you add.
10. Don’t forget to swirl the mixture after every couple of drops.
11. When a permanent light purple color change is apparent the end
point has been reached. If it starts turning blue you have gone
too far.
© 2010 Connections Education LLC. All rights reserved.
12. If it looks like the water solution created in procedure 5, you
have added too much NaHCO3.
13. Record the total number of drops of the sodium bicarbonate
solution you added to the vinegar in the Data Table.
14. Repeat step 7–13 two more times so that you will have
completed 3 titrations.
15. Use the average number of drops used to cause the reaction to
reach an endpoint for your final calculations.
16. Complete a formal lab report. Your report should include a
heading, an introduction explaining the purpose of the lab, all
data collected during the lab, and answers to the questions
below. Be sure to first type the question then the answer it.
Data Table:
# Trial
# of drops of
NaHCO
3
Volume of
NaHCO (mL)
3
1
Volume of
CH COOH (mL)
3
9.86 ml
2
9.86 ml
3
9.86 ml
average
9.86 ml
© 2010 Connections Education LLC. All rights reserved.
Questions:
1. Write the balanced equation for the reaction of acetic acid
( CH COOH) and sodium bicarbonate ( NaHCO ). What type of
3
3
reaction(s) took place?
2. Calculate the number of moles of NaHCO that were required to
3
neutralize the CH COOH in the vinegar.
3
3. Calculate the molarity of the vinegar sample. (Don’t forget to
convert mL to L.)
4. Calculate the number of grams of CH COOH in the vinegar.
3
5. Calculate the percent of acetic acid in the vinegar. (The density
of vinegar is 1.002 g/ml.)
6. Is it possible for the equivalence point of a titration to not be at
pH 7? Explain your answer.
7. What is the molarity of a CsOH solution if 30.0 mL of the solution
is neutralized by 26.4 mL 0.25 M HBr solution?
© 2010 Connections Education LLC. All rights reserved.










