Lab Techniques: Burners, Glassworking, Filtration
advertisement

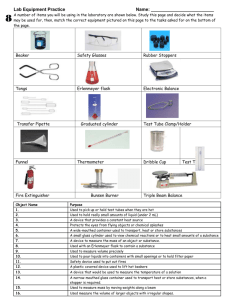
Laboratory Techniques Procedure: Laboratory Burners. Essentially all single-barrel burners used in chemical laboratories are Bunsen burners or some modification of that burner that was invented by the German chemist, Robert Bunsen (1811 – 1899) . One common burner used at Chemeketa C C is a modification of the Bunsen burner called a Tirrill burner (see below). Barrel Air vents Gas inlet Needle valve (for gas control) These burners make possible the complete combustion (nearly) of gaseous fuels by providing the proper ratio of fuel to air and the correct mixing of these gases in the barrel of the burner. Since this type of burner is used in many chemical experiments, it is important that you understand its operation and its adjustments in order to obtain the maximum efficiency. A flame is produced by the chemical reaction of two gases, a fuel and oxygen (in the air). The temperature of a flame is dependent mainly on two factors: the nature of the fuel, and on the rate and completeness of mixing and burning of the gases. Natural gas is composed largely of methane (CH4). Methane burns to form carbon dioxide and water in an unlimited supply of oxygen. The chemical equation for the reaction is: CH4 methane + 2 O2 oxygen CO2 + carbon dioxide → 2 H2O water 2 If oxygen is limited, considerable amounts of free carbon is formed. The very small particles of carbon are heated to incandescence. This produces a luminous flame or the yellow carbon flame. The equation for this reaction is: CH4 + O2 → C + 2 H2O In cases when there is insufficient oxygen combining with methane, some of poisonous gas carbon monoxide is formed. This is another example of the incomplete combustion of methane. The reaction showing this formation of carbon monoxide is: 2 CH4 + 3 O2 → 2 CO + 4 H2O First, let’s begin adjusting the gas supply and air inlet. These are both screw-type adjustments that do not allow for rapid adjustments. Connect the burner with the laboratory supply of natural gas by using a piece of rubber tubing. A burner is usually lit with the air vents nearly closed. The vents are closed by turning the barrel of the burner in a clockwise direction. After the gas has been turned on and lit, the size and the quality of the flame is adjusted by admitting air and regulating the flow of gas. Air is admitted by rotating the barrel; gas is regulated with the needle valve. Insufficient air will cause a bright yellow, smoky flame; too much air will cause the flame to be noisy and blow itself out. A Bunsen burner flame that is best for most purposes is shown below; such a flame is said to be nonluminous. The hottest part of the flame is just above the bright blue cone. Outer cone Region of highest temperature Bright blue cone (combustion zone) Inner dark cone 3 Glassworking. Place any broken glass in the container provided. Some of the experiments that you will do will require construction of simple glass apparatus. In preparing for these experiments, you will need to become familiar with the various manipulations of glass tubing. Improper techniques may result in an apparatus that does not work well, or may result in nasty cuts. (1) Cutting of glass tubes (or rods). Place on your lab bench desk top a piece of 6 mm glass tubing about 12 cm long. Make a deep scratch across the tube with one stroke using a triangular file. If the file is dull, several strokes may be required – all strokes should be in the same direction, do not saw the glass. Grasp the tube with both hands, turn the tube so that the scratch is away from you and your thumbs are opposite the scratch. Pull your arms back along side of your body and bend the tube outward. This, with a little practice will give a clean right angle break. Use the same procedure in cutting glass rods. (2) Fire-polishing. The glass tubes and rods that you have just cut need to have the sharp edges smoothed. Hold the ends of the glass tubes in your burner. Rotate the tube slowly until the glass starts to melt and the sharp edges are smooth. Do not heat the tubes so much that the opening becomes constricted. Careful! This glass is hot! (3) Bending Glass Tubing. Use a Fischer burner (see below right) and adjust the flame so that a sharply defined region of intense blue color is visible. Heat a piece of 6 mm glass tubing by rotating it in the flame until it begins to sag. When the glass tube feels flexible, remove the tube from the flame and bend it rapidly to 90º. Hold the bent tube at this angle until it hardens and then place it on an insulating surface (Ceramfab pad or wire gauze). Many poor bends are the result of uneven or excessive heating and failure to continue to rotate the tube in the flame. Practice making several bends of different angles and have the instructor check them. Some good and bad bands are shown below: good bends Fischer burner not so good bends 4 (4) Evaporation. Prepare a water bath by filling a 400 mL beaker about half full of water and placing the beaker on a hot plate. Turn the hot plate on high until the water boils, then turn the heat down keeping the water boiling at all times. You may have to add additional water to the beaker during the evaporation process. Cover the bottom of a standard test tube with a small amount of sodium chloride. Add distilled water until the test tube is about ¼ full and stir with a glass rod until the salt dissolves. Pour the sodium chloride solution into an evaporating dish and place the dish on top of the 400 mL beaker with the boiling water in it. If you have a small evaporating dish, you may have to use a smaller beaker so the dish fits without falling into the beaker. Heat the evaporating dish until all of the water has evaporated from the sodium chloride solution, leaving the sodium chloride as a residue. Flush the sodium chloride residue down the sink. (5) Filtration. Separating suspended solids from liquids is accomplished by the use of filters. Insoluble solids called residues or precipitates form during the course of some chemical reactions. In lab, these residues are separated from solutions by filtering them out on a paper filter. The liquid that passes through the paper is called the filtrate. Fill a regular test tube about ½ full of lead(II) nitrate solution. Fill a second test tube about ½ full of potassium chromate solution. Pour both test tube contents into a 100 or 150 mL beaker at the same time and observe the results. The yellow precipitate (residue) that forms is lead(II) chromate. Fold a circle of filter paper in half, then fold it in half again. Open the paper into a cone. Place the cone of filter paper into a funnel, wet the paper with distilled water to allow the paper to cling to the funnel and remain opened. Clamp the funnel and filter paper cone to a ring stand or support it with a iron ring (or just hold it). Place a small beaker beneath the funnel to catch the filtrate. Stir the yellow lead(II) chromate solution and pour it into the filter paper cone. Do not over-fill the filter paper cone. Lead(II) chromate is toxic and should not be flushed down the sink. So, after you have filtered all the yellow solution, use your forceps to remove the filter paper with the yellow residue on it from your funnel and place the paper and all in a waste jar on the lab supply cart. The rest of the yellow residue can be washed down the sink with your brush and some detergent. Forceps Forceps Waste Jar 5 Name Date Report for Experiment Laboratory Burners and Glassworking Articles Instructor’s Check Straight tubes Right-angle bends Questions & Problems 1. Why is it necessary to turn off the gas with the gas cock rather that with the valve on the burner? 2. Why is air mixed with gas in the barrel of the burner before the gas is burned? 3. How would you adjust a burner with: (a) has a yellow and smoky flame? (b) is noisy with a tendency to blow itself out? 6 4. Why are glass tubes and rods always fire-polished after cutting? Evaporation 5. What is the name (not formula) of the residue remaining after evaporation? Filtration 6. What is the name and color of the precipitate recovered by filtration? name (not formula) color 7. Why is the filter paper with the precipitate on it collected in a jar instead of thrown into the trash? 8. What is the main substance in the filtrate?