Adobe Acrobat Version of the Spring 2008 Exam 1 Key
advertisement

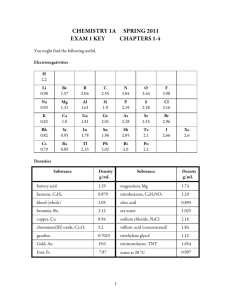
Chemistry 1A Spring 2008 Exam 1 Key Chapters 1-4 Electronegativities H 2.2 Li 0.98 Be 1.57 B 2.04 C 2.55 N 3.04 O 3.44 F 3.98 Na 0.93 Mg 1.31 Al 1.61 Si 1.9 P 2.19 S 2.58 Cl 3.16 K 0.82 Ca 1.0 Ga 1.81 Ge 2.01 As 2.18 Se 2.55 Br 2.96 Rb 0.82 Sr 0.95 In 1.78 Sn 1.96 Sb 2.05 Te 2.1 I 2.66 Cs 0.79 Ba 0.89 Tl 2.33 Pb 2.02 Bi 2.0 Po 2.2 Substance battery acid benzene, C6H6 blood (whole) bromine, Br2 chocolate copper, Cu chromium(III) oxide, Cr2O3 gasoline gold, Au Iron, Fe magnesium, Mg nitrobenzene, C6H5NO2 oleic acid phosphorus oxychloride, POCl3 sulfuric acid (concentrated) water at 20 °C Density in g/mL 1.29 0.879 1.05 3.12 1.75 8.94 5.2 0.7025 19.0 7.87 1.74 1.20 0.895 1.675 1.84 0.997 1 Xe 2.6 Answer the following by writing the word, words, letter, letters or number in each blank that best completes each sentence. (1½ point each blank unless stated otherwise) 1. A(n) heterogeneous mixture is a mixture with two or more phases. 2. Nanoscale means on the order of the size of atoms. 3. Metalloids or semimetals are the elements that have some but not all of the characteristics of metals. 4. The accepted SI standard for amount of substance is the mole, which has an abbreviation of mol. 5. The accepted SI base unit for energy is the joule, which has an abbreviation of J. (2 points) 6. The elements in group 1 on the periodic table are called alkali metals. 7. The SI (metric) prefix associated with 10−12 is pico, which has an abbreviation of p. (2 points) 8. The common units associated with the density of gases are g/L. 9. The bond between a carbon atom and an oxygen atoms is polar covalent (nonpolar covalent, polar covalent, or ionic). 10. In the H-F polar covalent bond, the H is partial positive, and the F is partial negative. (2 points) 11. A(n) cation is an ion formed from an atom that has lost one or more electrons and thus has become positively charged. 12. Atomic number is the number of protons in an atom’s nucleus. It establishes the element’s identity. 13. A(n) ionic hydrate is an ionic compounds with water molecules trapped within the crystal lattice. 14. A(n) oxyanion is a polyatomic ions with the general formula HaXbOcd−. (The a can be 0.) 15. Alkanes are hydrocarbons (compounds composed of carbon and hydrogen) in which all of the carbon-carbon bonds are single bonds. 2 16. Draw a reasonable Lewis structure for C2H3F. (4 points) 17. Draw Lewis structures for all of the constitutional isomers of C4H10. 18. Identify each of the following as a binary covalent compound, a binary ionic compound, a binary acid, an ionic compound with a polyatomic ion, an oxyacid, an alcohol, or a sugar. Write name for each. (8 points) Chemical Formula Type of Substance Name NF3 binary covalent nitrogen trifluoride Mg(H2PO2)2 ionic with polyatomic ion magnesium dihydrogen hypophosphite Cr(CN)3 ionic with polyatomic ion chromium(III) cyanide HClO4 oxyacid perchloric acid 19. Identify each of the following as a binary covalent compound, a binary ionic compound, a binary acid, an ionic compound with a polyatomic ion, an oxyacid, an alcohol, or a sugar. Write formula for each.) (8 points) Chemical Formula Type of Substance Formula hydrogen iodide binary covalent HI ferrous sulfite ionic with polyatomic ion FeSO3 ethanol alcohol C2H5OH hydrosulfuric acid binary acid H2S(aq) 3 20. The name that corresponds to the following structure is 2,4-dimethylhexane. (3 points) 21. Write a complete, balanced equation for the combustion of solid amino acid methionine, C5H11NSO2. The nitrogen in this compound goes to N2(g). (6 points) 2C5H11NSO2(s) + 31/2O2(g) 10CO2(g) + 11H2O(l) + 2SO2(g) + N2(g) or 4C5H11NSO2(s) + 31O2(g) 20CO2(g) + 22H2O(l) + 4SO2(g) + 2N2(g) 22. Complete the following table by (1) writing the name for the type of particle viewed as forming the basic structure of each of the following substances and (2) writing the name of the type of attraction the is broken when these substances are melted or boiled, e.g. covalent bonds, dipole-dipole attractions, etc. (1 point each box) Substance Particles to Visualize Type of Attraction C6H14 molecules London forces CH3NH2 molecules hydrogen bonds and London forces hydrogen chloride molecules dipole-dipole attractions and London forces chlorine molecules London forces Carbon in the diamond form atoms covalent bonds xenon atoms London forces NaNO3 Na+ cations and NO3− anions ionic bonds manganese cations in a sea of electrons metallic bonds 4 For the following numerical problems, be sure to carefully show your work and round your answer using the appropriate guidelines. NOTE: Remember that there is part credit for each problem. Even if you cannot do all of a problem, be sure to set up as much of it as you can. (6 points each) 23. Phosphorus oxychloride, POCl3, is a colorless, fuming liquid used to make gasoline additives. What mass of chlorine in megagrams is contained in 4.765 m3 of POCl3? ⎛ 103 L ⎞⎛ 103 mL ⎞ ⎛ 1.675 g ⎞ ⎛ 1 mol POCl 3 ⎞ ⎛ 3 mol Cl ⎞ ⎛ 35.4527 g Cl ⎞ ⎛ 1 Mg ⎞ ? kg Cl = 4.765 m3 POCl 3 ⎜ ⎟⎜ ⎟⎜ ⎟⎜ ⎟ ⎜ 6 ⎟ = 5.536 Mg Cl ⎟⎜ 3 ⎟⎜ ⎝ 1 m ⎠⎝ 1 L ⎠ ⎝ 1 mL ⎠ ⎝ 153.331 g POCl 3 ⎠ ⎝ 1 mol POCl 3 ⎠ ⎝ 1 mol Cl ⎠ ⎝ 10 g ⎠ 24. The FeS2 found in pyrite ore is a source of sulfur for the production of sulfuric acid. The following equation describes the first step in this process. 4FeS2 + 11O2 → 2Fe2O3 + 8SO2 How many kilograms of pyrite ore that is 92.6% FeS2 are necessary to produce 48.95 pounds of iron(III) oxide, Fe2O3 in the first step of this process? ⎛ 453.6 g ⎞ ⎛ 1 mole Fe2 O3 ⎞ ⎛ 4 mole FeS2 ⎞ ⎛ 119.977 g FeS2 ⎞ ⎛ 1 kg ⎞ ⎛ 100 kg ore ⎞ ? kg ore = 48.95 lb Fe2 O3 ⎜ ⎟⎜ ⎟⎜ ⎟ ⎟⎜ 3 ⎟⎜ ⎟⎜ ⎝ 1 lb ⎠ ⎝ 159.69 g Fe2 O3 ⎠ ⎝ 2 mole Fe2 O3 ⎠ ⎝ 1 mole FeS2 ⎠ ⎝ 10 g ⎠ ⎝ 92.6 kg FeS2 ⎠ or ⎛ 4 x 119.977 lb FeS2 ? kg ore = 48.95 lb Fe2 O3 ⎜ ⎝ 2 x 159.69 lb Fe2 O3 5 ⎞ ⎛ 100 lb ore ⎞ ⎛ 1 kg ⎞ ⎟⎜ ⎟⎜ ⎟ = 36.0 kg ore 92.6 lb FeS 2.205 lb ⎝ ⎠ ⎝ ⎠ 2 ⎠ Answer the following in short answer form. 25. Write a description of the element nitrogen as it is found at room temperatures and pressures. Your description should include mention of the particles that form its structure, whether it’s a gas, liquid, or solid, how the particles are moving, how much space they occupy, and the strengths of attractions between the particles. Nitrogen is composed of diatomic molecules, N2, with seven protons and a +7 charge in each of the two nuclei surrounded by a −14 electron-charge cloud. Nitrogen is a gas at room temperature and pressure so the particles occupy only a small volume within the container (about 0.1%), which leaves them relatively far apart (about ten times their diameter). This leads to very weak attractions between the particles allowing them to move freely in straight-line paths interrupted by collisions between molecules that lead to changes in direction and velocity. 26. Write an explanation for why an excess of one or more of the reactants are added in a chemical reaction and explain why other reactants are limiting. There are four reasons for choosing some reactants to be limiting and others to be in excess. • Expense…the more expensive reactant is more likely to be limiting. • Importance…the more important reactant is more likely to be limiting. • Danger…the more dangerous reactant is more likely to be limiting. • Ease of separation…the reactant that is easier to separate from the product mixture is more likely to be added in excess. 6