Chem1stSem FinalExamStudyGuideTimberlake
advertisement
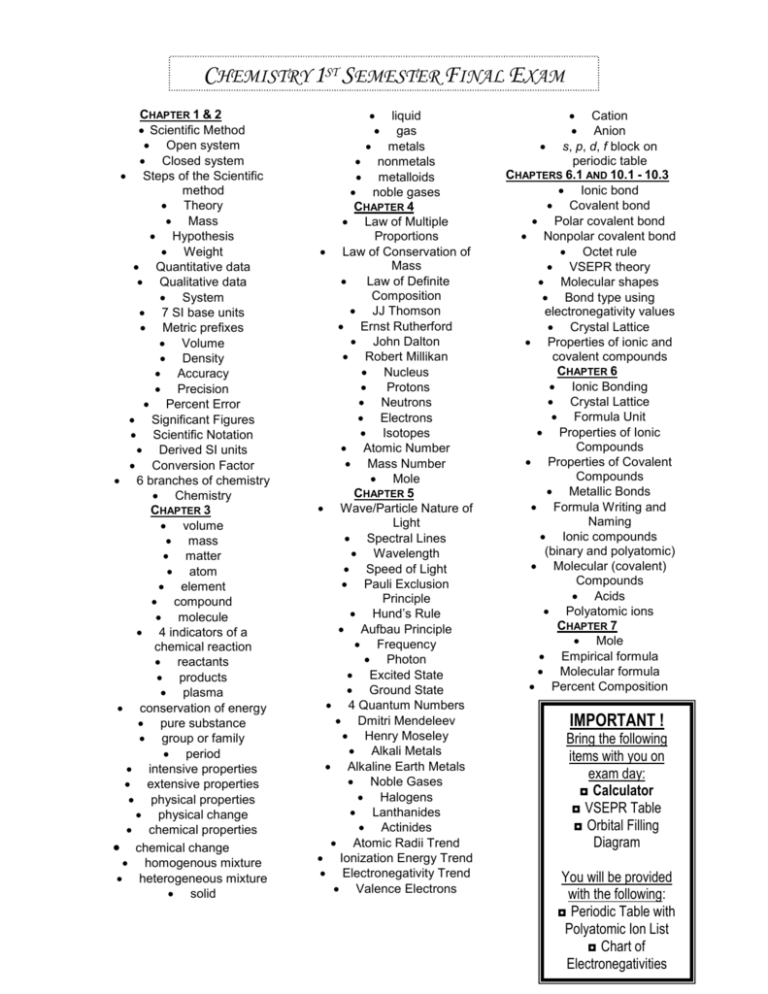
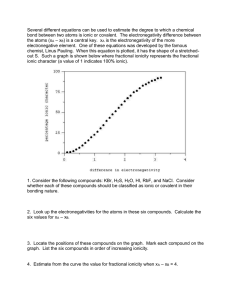
CHEMISTRY 1ST SEMESTER FINAL EXAM STUDY Gliquid UIDE CHAPTER 1 & 2 Scientific Method Open system Closed system Steps of the Scientific method Theory Mass Hypothesis Weight Quantitative data Qualitative data System 7 SI base units Metric prefixes Volume Density Accuracy Precision Percent Error Significant Figures Scientific Notation Derived SI units Conversion Factor 6 branches of chemistry Chemistry CHAPTER 3 volume mass matter atom element compound molecule 4 indicators of a chemical reaction reactants products plasma conservation of energy pure substance group or family period intensive properties extensive properties physical properties physical change chemical properties chemical change homogenous mixture heterogeneous mixture solid gas metals nonmetals metalloids noble gases CHAPTER 4 Law of Multiple Proportions Law of Conservation of Mass Law of Definite Composition JJ Thomson Ernst Rutherford John Dalton Robert Millikan Nucleus Protons Neutrons Electrons Isotopes Atomic Number Mass Number Mole CHAPTER 5 Wave/Particle Nature of Light Spectral Lines Wavelength Speed of Light Pauli Exclusion Principle Hund’s Rule Aufbau Principle Frequency Photon Excited State Ground State 4 Quantum Numbers Dmitri Mendeleev Henry Moseley Alkali Metals Alkaline Earth Metals Noble Gases Halogens Lanthanides Actinides Atomic Radii Trend Ionization Energy Trend Electronegativity Trend Valence Electrons Cation Anion s, p, d, f block on periodic table CHAPTERS 6.1 AND 10.1 - 10.3 Ionic bond Covalent bond Polar covalent bond Nonpolar covalent bond Octet rule VSEPR theory Molecular shapes Bond type using electronegativity values Crystal Lattice Properties of ionic and covalent compounds CHAPTER 6 Ionic Bonding Crystal Lattice Formula Unit Properties of Ionic Compounds Properties of Covalent Compounds Metallic Bonds Formula Writing and Naming Ionic compounds (binary and polyatomic) Molecular (covalent) Compounds Acids Polyatomic ions CHAPTER 7 Mole Empirical formula Molecular formula Percent Composition IMPORTANT ! Bring the following items with you on exam day: ◘ Calculator ◘ VSEPR Table ◘ Orbital Filling Diagram You will be provided with the following: ◘ Periodic Table with Polyatomic Ion List ◘ Chart of Electronegativities