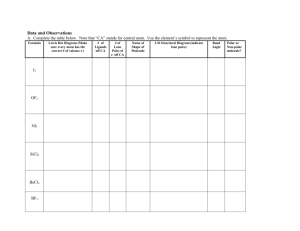
Shapes of Molecules We must keep in mind that molecules are three
advertisement

Shapes of Molecules We must keep in mind that molecules are three-dimensional. We need to translate Lewis Dot or structural diagrams into 3D The Lewis Dot and structural diagrams require identification of the central atom(s) and places other atoms around it to match up bonding electrons. This does not show actual location of the valence electrons. Position of the electrons is determined by the rule LIKE CHARGES REPEL VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) - Assumes that groups of negative valence electrons spread out as far as possible - Actual shapes have been confirmed by Xray technology ****** LONE PAIRS INFLUENCE THE SHAPE OF THE MOLECULE Predicting Shapes About Central Atoms in Molecules - Uses the electron dot diagram and the VSEPR theory THERE ARE FIVE SHAPES POSSIBLE: SHAPE NUMBER OF LONE PAIRS NUMBER OF BONDING GROUPS* LINEAR 0 2 BENT (V-SHAPE) 2 2 PYRAMIDAL 1 3 TETRAHEDRAL 0 4 TRIGONAL PLANAR 0 3 * Single, double, triple, bonds each counts as one bonding group Examples: Linear molecules have 2 bonding groups and no lone pairs on the central atom, so the bonding groups will be as far apart as possible, forming a straight angle . All diatomic molecules are linear, so is CO2 Bent molecules have two bonding groups and two lone pairs on the central atom. electron groups as far apart as possible. Water is the best example. This pushes all Pyramidal shapes are due to one lone pair on the central atom with three bonding groups. example is ammonia, NH3 Tetrahedral shapes have 4 bonding groups without any lone pairs. A good A good example is methane, CH 4 The final shape is trigonal planar, which happens in cases where there are two central atoms, each with three bonding groups attached to it and no lone pairs. C2H2, ethene. DRAWING 3D SHAPES IN 2D Linear Examples - (not a problem) Bent Examples - (not a problem) Trigonal Planar Examples - (not a problem) Pyramidal Examples - Note the use of special lines that show the bond coming out or going back behind. Tetrahedral Examples - TRY THESE: (a) SiCl4 (b) C2F2 (c) CS2 (d) SeFCl Polar and Non-Polar Bonds Not all non-metals have the same electronegativity. Any time there is a difference between two atoms, there is a polar bond set up. This means one atom in the bond will be slightly negative, because it has the higher electronegativity and the electrons will be pulled more toward it. The other end will be slightly positive. Non-polar covalent bonds: * all diatomic elements have non-polar bonds * different elements with the same electronegativity will also be non-polar Polar bonds: * the greater the difference between the two the stronger the polarity Classify these bonds: C-H Br-Br C-O H-O Polar Molecules In order to determine whether a molecule is polar we need: $ to know whether there are differences in electronegativity forming molecular dipoles $ the Lewis Diagram $ the VSEPR Theory We can look at the shape of the molecule, and write in all the bonds that are polar and which way the electrons will be pulled. If the molecular dipoles cancel each other out, there is no overall polarity of the molecule. Example: there will be differences between C and O in CO2, but they will pull equally in opposite directions so they cancel out Example: there are differences in the electronegativities of H and O in water. Since water is a bent shape, the polar bonds will not cancel each other out and the entire molecule is polar. Determine these: SI2, NH3, CH4, CH2Cl2 To sum up: Draw Lewis Dot Diagram And Shape Diagram Non-polar Bonds Only Polar Bonds Present Symmetrical Around the Central atom Non-polar Molecule Non-symmetrical around the central atom Non-polar Molecule Polar Molecule