CP Chemistry Name
advertisement

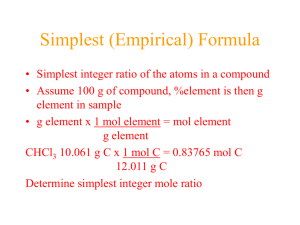
C.P. Chemistry Name: ______________________ Review Sheet Date: _______________________ Chapter 7: The Mole and Chemical Quantities Please answer each question as completely as possible and include all the work along with units. 1) What is the molar mass of the following: (you must show work for compounds) MgCl2 95.3 g/mol Rn 222 g/mol C11H22O12 346 g/mol H3PO4 98 g/mol (NH4)2SO4 132.1 g/mol Co 58.9 g/mol 2) Determine the percent composition of the elements in the following compounds a. N2O 63.6% N, 36.4% O b. Ca(OH)2 54.1% Ca, 43.2% O, 2.7% H c. C14H10O4 69.4% C, 4.1% H, 26.4% O d. C7H5NO3S45.9% C, 2.7% H, 7.6% N, 26.2% O, 17.5% S Fill in the blanks in chart by using the appropriate computation. Please show all work in the space provided and remember units. Compound Number of Moles Li(OH) 5.57 moles C5H11O7 Mg2(PO4)3 CuSO4 d) 3.58 mol 34.56 moles h) 0.627 mol Molar Mass a) 23.9 g/mol b) 133 g 183.11 g/mole f) 333.6 g/mol i) 159.6 g/mol Mass 654.7 grams g) 1.15x104g 100 grams Representative Particles c) 3.35x1024 e) 2.15x1024 5.66 * 1021 atoms j) 3.77x1023 3) Name the following hydrates or give the formula: a. Na2CO3 * 10H2O sodium carbonate decahydrate c. CaSO4 * 2H2O calcium sulfate dihydrate e. Magnesium Chloride heptahydrate MgCl2 · 7H2O b. BaCl2 * 4H2O barium chloride tetrahydrate d. Copper Sulfate trihydrate CuSo4 · 3H2O 4) Calcium bromide is a compound used in the manufacture of fire extinguishing materials. Analysis reveals that calcium bromide contains 20% calcium and 80% bromine. Calculate the empirical formula. CaBr2 5) What is the empirical formula of a compound if the percent composition is aluminum 15.77%, sulfur 28.11% and oxygen 56.12%? Al2S3O12 6) If the molar mass of a compound is 108g/mole and it contains 4.02g N and 11.48 g O, what is its molecular formula? Empirical = Molecular = N2O5 7) The molar mass of a compound is 92g/mole. Analysis of the compound shows 0.608g of N and 1.388g O. What is the molecular formula of this compound? Empirical Formula: NO2 Molecular Formula: N2O4 8) Beryl is a hard mineral which occurs in a variety of colors. A sample contains 2.52 g Be, 5.01g Al, 15.68g Si, and 26.79g O. Determine the empirical formula for Beryl. Be3 Al2 Si6 O18 9) Determine the molecular formula for ibuprofen, a common headache remedy. Ibuprofen contains a percent composition of 75.7% C, 8.80% H and 15.5% O and has a molar mass of 206g/mole. Empirical Formula = Molecular Formula = C13 H18 O2 10) What is the density of O2 at STP? 1.43 g/L 11) 3 L of a gas has the mass of 2.00 g. What is the molecular mass? 12) What volume does 22.0 g of CO2 at STP occupy? 11.2 L 14.9 g/mol