6.7 & 6.9 Empirical & Molecular Formulae
advertisement

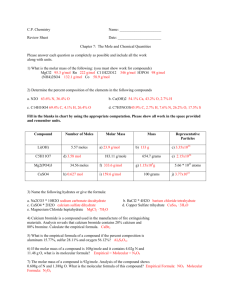
6.7 & 6.9 Empirical & Molecular Formulae pp. 289 – 293, 296 - 300 Empirical & Molecular Formulae • The chemical word equation for the reaction between aluminum and bromine liquid is as follows: aluminum + bromine liquid → aluminum bromide • Its balanced chemical equation is: 2Al(s) + 3Br2(l) → Al2Br6(s) Empirical & Molecular Formulae • At first glance, the formula for aluminum bromide in the equation, Al2Br6 appears to be a strange one. • Aluminum’s valence is 3 and bromine’s valence is 1 and using the cross-over rule, the formula would end up as being AlBr3. • However, through chemical analysis, the molecular formula is Al2Br6. • The molecular formula is the exact formula of the compound. • The empirical formula is the simplest whole number ratio of the elements’ atoms in a compound. – The empirical formula of aluminum bromide is AlBr3. • In many cases, the empirical formula is the molecular formula of a compound. – Water, H2O, is such a case. • The elements’ respective subscripts in the formula show a mole ratio relationship. • The formula for water, H2O, shows us that on a macroscopic level, there are 2 moles of hydrogen for every 1 mole of oxygen atoms. • To calculate an empirical or a molecular formula for a compound, the given masses for each element must be converted into their respective whole number mole values. Sample Calculation • What is the empirical formula of a compound which is found by analysis to contain 2.2% hydrogen, 26.7% carbon and 71.1% oxygen? • If we assume a 100g sample, G: mH = 2.2g mC = 26.7g R: Empirical Formula mO = 71.1g CHO = ? A: n = m ÷ M S: nH = 2.2 g ÷ 1.01 g/mol = 2.18 mol nC = 26.7 g ÷ 12.01 g/mol = 2.22 mol nO = 71.1 g ÷ 16.00 g/mol = 4.44 mol Mole Ratio: H 2.18 mol : C : 2.22 mol : O : 4.44 mol Dividing each by the lowest mole value, 2.18 1 mol : 1.02 mol : 2.04 mol If the values are within 0.1 of a whole number, the numbers can be rounded off to the nearest whole number 1 mol : 1 mol : 2 mol Therefore, the empirical formula is HCO2 Example # 2 • A hydrocarbon, upon analysis, shows the following mole to mole ratio relationship between carbon and hydrogen Mole Ratio: C 0.166 mol : H : 0.444 mol Dividing each by the lowest mole value, 0.166 1 mol : 2.67 mol 2.67 cannot be rounded, so try multiplying each number by 2 2 mol : 5.3 mol 5.3 cannot be rounded, so try multiplying each number from the previous page by 3 3 mol : 8.02 mol Round each to the nearest whole number 3 mol : 8 mol Therefore, the empirical formula is C3H8 Molecular Formulae • The molecular formula of a compound gives you the actual composition of a molecule. • Its calculation is not much different from that of the empirical formula. • Once you calculate the empirical formula you need to do one further calculation: x = Mcompound ÷ Mempirical formula • You will then multiply each subscript in the empirical formula by x Sample Calculation • The empirical formula of a compound is determined to be CH. Its molecular molar mass is 104.16 g/mol. Determine its molecular formula. G: MCH = 13.02 g/mol Mcompound = 104.16 g/mol R: Molecular formula of compound A: x = Mcompound ÷ Mempirical formula S: x = 104.16 g/mol ÷ 13.02 g/mol =8 P: Therefore the molecular formula is C8H8 Homework • Read pp. 289 – 292 – Answer # 1 – 11 p. 293 • Read pp. 304 – 305 – Answer # 1 – 9 p. 300