Clincal Quiz - McMaster University Medical Journal
advertisement
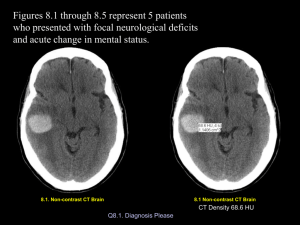
47 Clincal Quiz CASES IN NEURORADIOLOGY Jason Martin1 Michael G. DeGroote School of Medicine, McMaster University, 1 Hamilton, Ontario, Canada Author for Correspondence: Jason Martin Michael G. DeGroote School of Medicine, McMaster University, 1280 Main Street West, MDCL 3010, Hamilton, ON L8S 4L8 Email: jason.martin@medportal.ca Conflict of Interest: The authors have no conflicts of interest to disclose. CASE 1 A CASE 2 67 year-old woman presents to the emergency department with loss of consciousness. The patient’s daughter mentions that her mother lost consciousness hours after hitting her head on her car door while exiting the vehicle. She has a past medical history of atrial fibrillation, diabetes type II, remote myocardial infarction, hypertension and dyslipidemia. She is currently taking Aspirin, Warfarin and Insulin. The emergency physician ordered a non-contrast axial computed tomography (CT) scan, shown below (Figure 1). Paramedics brought a 20 year-old female in to the hospital after a motorcycle accident. Upon examination, the patient is found to have a fractured right humerus and left femur. Glasgow Coma Scale (GCS) was four. The accident report describes a high-speed impact with the patient being ejected from the vehicle during the collision. The patient was not wearing a helmet. CT angiography was ordered by the emergency physician, shown below (Figure 2). There is no past medical history. Patient is currently taking the Alysena oral contraceptive pill. Figure 1. Axial CT images in a 67 year-old woman with low-impact head injury. Figure 2. CT Angiogram (with IV contrast) of a 20 year-old female involved in a motor vehicle accident (MVA). Sagittal (a) and coronal (b) slices are shown. What is the most likely diagnosis? A. Contusion B. Subdural Hematoma C. Epidural Hematoma D. Subarachnoid Hemorrhage The above process can best be described as: The patient’s imaging is least consistent with which of the following diagnoses? A. Dissection B. Carotid Embolism C. Brain Death D. All would be on your differential A. Acute B. Subacute C. Chronic D. None of the above WWW.MUMJ.ORG Clinical Quiz 48 McMASTER UNIVERSITY MEDICAL JOURNAL Cerebral angiography (Figure 3) was performed to confirm the diagnosis. This entity can occur via genetic mutations. True False What type of pathology is this? A. Sporadic B. Acquired C. Familial Figure 3. Catheter cerebral angiography of Internal Carotid Artery (ICA) with radiopaque dye. ANSWERS What is the most likely diagnosis? CASE 1 A. Dissection B. Carotid Embolism C. Brain Death Answers: B, C CASE 3 A 73 year-old female presents to her family doctor with rapidly progressive dementia. Over the past few weeks, the patient has experienced increasing memory loss, hallucinations, ataxia, unsteady gait, seizures and myoclonus. She has no past medical history. She is on no medication currently. Her family physician orders an urgent MRI to elucidate the cause of her symptoms, shown below (Figure 4). Figure 1. Axial CT images in a 67 year-old woman with low-impact head injury. Arrows reveal a subural hematoma in the left fronto-parietial region. Explanation Figure 4. Axial MR images of a 53 year-old woman with rapidly progressing dementia. Diffusion weighted imaging (DWI) (a) and T2 (b) images are shown here. What is the likely diagnosis? A. Leigh Syndrome B. Osmotic Demyelination Syndrome C. Wilson Disease D. Hypoxic Ischemic Injury E. Creutzfeld-Jakob Disease VOLUME 11 NO. 1 | 2014 A subdural hematoma is the most likely diagnosis for this patient’s condition, and is characterized by an extra-axial, crescent-shaped hematoma (blue arrows in Figure 1). The patient’s age (>60 years) makes a subdural hematoma more likely as the dura is more tightly adhered to the skull in this population. The hematoma is hypodense relative to cortex, suggesting a chronic bleed. The anticoagulation status of the patient should increase the clinician’s suspicion for a vascular disruption. Acute hematomas in most patients consist primarily of coagulated blood, which is hyperdense relative to brain parenchyma on CT. Over time, resorption of the clot and neovascularization results in increasing isodensity and, later, hypodensity of the hematoma, assuming re-bleeding does not occur. Etiology Subdural Hematoma (SH) - Subdural hematomas are typically caused by tearing of the bridging cortical veins as they cross the subdural space,1 usually secondary to shearing forces resulting from a sudden 49 CASE IN NEURORADIOLOGY change in cranial velocity. The arachnoid may also be torn, creating a mixture of blood and CSF in the subdural space.1 Figure 5 outlines the basic anatomy of the skull and meninges. spontaneous acute subdural hematomas are associated with an underlying abnormality that may precipitate hemorrhage, such as dural arteriovenous fistulas or cerebral tumors. Epidural Hematoma (EH) – Trauma to the sphenoid resulting in rupture of the middle meningeal artery is the most common etiology for epidural hematomas, although the anterior meningeal artery is infrequently involved. An associated skull fracture is present in approximately 80% of cases.1 Epidural hematomas can often be homogenous, and chronic, hypodense epidural hematomas are also seen. Associated pain results from shearing of the dura from bone.1. The increased incidence of epidural hematomas in younger patients is not only a function of the increased incidence of head injury in that population, but also related to the dural structure.1 In younger patients, the Dura is less tightly attached to the skull, creating a favourable environment for hematoma formation. Epidural Hematoma - Epidural hemorrhages are most often precipitated by a blunt force impact with or without loss of consciousness. Patients with an initial “lucid” interval may suffer rapidly deteriorating neurological status with severe headache and progressive loss of consciousness. Other symptoms of elevated intracranial pressure (ICP) are the result of mass effect, and include nausea, vomiting, headache and visual disturbances. Cushing’s triad can be seen in elevated ICP, and involves an increased systolic blood pressure, bradycardia, and an abnormal respiratory pattern. If elevated ICP progresses without intervention, it may lead to death. Subarachnoid Hemorrhage (SAH) – In 85% of cases of spontaneous SAH, the cause is rupture of a cerebral aneurysm—a weakness in an arterial wall (specifically, the medial layer) that becomes enlarged. They tend to be localized at bifurcation points in the circle of Willis and its branches. While most cases of SAH are due to bleeding from small aneurysms, larger aneurysms are more likely to rupture.2 An initial angiogram may fail to detect an aneurysm in 15–20% of spontaneous SAH.3 Half of these undetected cases are a result of non-aneurysmal perimesencephalic hemorrhages, in which blood is limited to the subarachnoid spaces within the midbrain. The source of bleeding in these cases is uncertain.2 Other common causes of spontaneous SAH include arteriovenous malformations, vasculopathies, and hemorrhagic neoplasms.2 Less frequently, SAH may result from cocaine abuse, sickle cell anemia, anticoagulant therapy, and pituitary apoplexy.3,4 Subarachnoid Hemorrhage - Patients characteristically present with a thunderclap headache, often reported by patients as the worst headache of their lives. Photophobia is common, which overlaps with the presentation of meningitis. Consciousness may be lost acutely in up to 50% of patients.5. Imaging Epidural Hematoma – on CT, EH are typically bi-convex or lentiform in shape, and most frequently occur beneath the squamous aspect of the temporal bone (Figure 7a). They are hyperdense, somewhat heterogenous, and sharply demarcated. Depending on their size, secondary features of mass effect (e.g. midline shift, subfalcine herniation, uncal herniation) may be present.1 Subdural Hematoma generally presents as a crescent-shaped, extraaxial, multi-septated collection of fluid on CT. Over 85% are unilateral1. SDHs do not respect suture lines, but are limited by dural reflections, such as the falx cerebri, tentorium, and falx cerebelli. Ascertaining the temporal sequence (timing) of the bleed is aided by examining the density of the hematoma on a non-contrast enhanced CT (Figure 6). Time After Injury Appearance on CT Acute Figure 5. Layers of the skull and meninges. < 72 Hours Hyperdense Subacute 3-7 Days Isodense or Hypodense Chronic > 3 Weeks Hypodense, but may be mixed when rebleeding has occurred Clinical History Subdural Hematoma– Acute subdural hematomas usually present in the setting of head trauma. This is especially the case in young patients, where they commonly present in addition to cerebral contusions. Most patients present with decreased level of consciousness, with abnormal pupillary response in 30 - 50% of cases.1 Occasionally, Figure 6. Relationship of timing of bleed and CT attenuation characteristics. WWW.MUMJ.ORG Clinical Quiz 50 McMASTER UNIVERSITY MEDICAL JOURNAL Subarachnoid Hemorrhage - The sensitivity of CT to the presence of subarachnoid blood is strongly influenced by both the amount of blood and time of onset. The diagnosis is suspected when hyperattenuating collections infiltrate the subarachnoid space. Most commonly this is adjacent to the circle of Willis, largely because the majority of berry aneurysms form in this region (~65%), or in the sylvian fissure (~30%).1 Small amounts of blood can sometimes be appreciated pooling in the interpeduncular fossa, appearing as a small hyperdense triangle, or within the occipital horns of the lateral ventricles. Figure 2. CT Angiogram (with IV contrast) of a 20 year-old female in a MVA. Sagittal (a) and coronal (b) slices are shown. (c) Noncontrast CT, axial view. Arrows reveal lack of perfusion within the internal carotid artery. Brain Death - Imaging General radiologic features include sulcal and gyral effacement, ventricle effacement and compression.6 Figure 7. Spectrum of vascular and parenchymal involvement in head trauma. Epidural hematoma (a), subdural hematoma (b) and subarachnoid hemorrhage (c) are shown here. The need for radiological characterization and diagnosis affects treatment choices for the patient in both the acute and chronic setting. With the history of head trauma, the need for surgical evacuation in EH is often more time-sensitive in SDH or SAH. With timely recognition and intervention, patient morbidity and mortality may be improved. CASE 2 Answers: D, C CT – Look for diffuse edema, swollen gyri and compressed cisterns & ventricles, and no enhancement of vasculature with contrast. MRI - T1 reveals hypointense loss of grey-white matter differentiation. Brain herniation may be present. T2: Reveals hyperintense cortex and swollen gyri. MR Angiography: May show minimal or absence of intracranial flow. A secondary sign known as the “hot nose” of brain death can be seen on scintigraphic brain flow scans (Figure 8).7 The hot nose sign may be seen in patients who have diminished blood flow in one or both internal carotid arteries.7 Decreased internal carotid blood flow leads to increased or collateral flow through the external carotid artery on the involved side, producing markedly increased perfusion to the nasal region.7 Explanation Given the history of a high-grade head impact and the appearance of a smoothly tapered internal carotid artery with absence of intracerebral perfusion on CT angiogram, this patient’s imaging is consistent with brain death. Brain Death - Pathophysiology Brain death is the culmination of severe cellular and extracellular edema in the context of raised intracranial pressure (ICP).1 Markedly elevated ICP causes low cerebral blood flow. If ICP is higher than end-diastolic pressure, retrograde flow occurs.1 If ICP is higher than systolic pressure, cessation of blood flow occurs, leading to complete and irreversible loss of brain function.1 In the majority of instances, clinical diagnosis suffices in classifying a patient as brain dead. Conformation is usually performed with CTA, MRA or cerebral angiography. However, before the diagnosis is even confirmed, preliminary CT or MR studies may reveal characteristic changes, especially when ordered in the setting of trauma. VOLUME 11 NO. 1 | 2014 Figure 8. Hot Nose Sign. 51 CASE IN NEURORADIOLOGY In this current case, both carotid dissection and embolism would remain on the differential. A. Dissection – a possibility, albeit the odds of bilateral internal carotid dissection are low. A history of trauma should raise suspicion for dissection, but the angiographic appearance is quite different, often demonstrating abnormal vessel contour, a visible psuedoaneurysm, or the string sign, a thin stripe of flow caused by decreased pressure distal to the stenosis1. B. Carotid Embolism – A valid diagnostic consideration, but again the odds of a concomitant embolism in the same section bilaterally are very low. Embolic lesions also typically present with an abrupt tapering of the occluded vessel, with clinical signs of infarction to the non-perfused brain region. CASE 3 Answers: E, FALSE, A Explanation There is considerable overlap in presentation between the listed differential. However, a characteristic feature in this patient provides a clue to diagnosis. In this case, the T1 hyperintensity of the globus pallidus suggest sporadic CJD, and the T2 hyperintense basal ganglia and thalami on MRI further support the diagnosis of CJD. These findings, combined with the clinical picture of rapidly progressing dementia in a middleaged female make CJD the likely diagnosis. Pathophysiology Creutzfeldt–Jakob disease (CJD) is a degenerative neurological disorder that is incurable and lethal. It is the most common form of the human spongiform encephalopathies. CJD results from accumulation of misfolded versions of the prion protein encoded by the prion protein (PrP) gene on chromosome 20. In CJD, the brain tissue atrophies, creating holes and taking on a sponge-like appearance. Gross cerebral atrophy may result1. Prions are misfolded proteins, which propagate by converting their properly folded versions. Acquired CJD is better encapsulated by the terms PrPi (Iatrogenic) and variant CJD (vCJD). vCJD is thought to be caused by consumption of food contaminated with prions. Iatrogenic CJD arises from contamination from an infected person, classically associated with human-derived pituitary growth hormone, corneal and/or meningeal transplants. CJD can also be acquired via introduction of prions into healthy cells (1% of cases), or sporadically from mutations of the normal host-encoded protein (85% of cases). 1 Figure 4. Axial MR images of a 53 year-old woman with rapidly progressing dementia. Diffusion weighted imaging (DWI) (a) and T2 (b) images are shown here. Arrows reveal DWI hyperintensity of the globus pallidus and T2 hyperintensity of the basal ganglia and thalami. Imaging CT – Usually normal, but may show progressive atrophy and ventricular dilatation. MR – T1 – Caudate or globus pallidus hyperintensity may be reported in sporadic CJD. T2 – Hyperintense basal ganglia and thalami may be seen, as well as hyperintense cortical grey matter and atrophy. FLAIR – The pulvinar sign suggests CJD: Bilateral symmetrical hyperintensity of pulvinar (posterior) nuclei of thalamus relative to anterior putamen. The hockey stick sign shows symmetrical pulvinar and dorsomedial thalamic nuclear hyperintensity, characteristic of variant CJD. A. Leigh Syndrome - Leigh disease is one of many mitochondrial disorders, due to a wide variety of possible genetic mutations in mitochondrial DNA (mtDNA). As with any mitochondrial disorder, it is only inherited from the mother. Leigh Syndrome primarily presents in children, with psychomotor delay and superimposed signs of basal ganglia and brainstem dysfunction (ataxia, ophthalmoplegia, dystonia, cranial nerve palsies).8 B. Osmotic Demyelination Syndrome - acute demyelination of the white matter tracts traversing the pons. It seen in the setting of osmotic changes, typically with rapid correction of hyponatraemia. Lesions are hyperintense on T2 sequence MRI imaging, most often in the putamen and external capsule. C. Wilson Disease (WD) – is a disorder that results from abnormal ceruloplasmin metabolism secondary to mutations in the ATP7B gene located on chromosome 13q14.3. This gene product is a cation transport ATPase, functioning to export copper out of cells. When mutated, the dysfunctional gene product leads to total body copper elevation, with deposition and resultant damage to a variety of organs, notably the liver and brain. If copper predominates, affected organs WWW.MUMJ.ORG Clinical Quiz 52 McMASTER UNIVERSITY MEDICAL JOURNAL are T1 hyperintense and T2 hypointense. If gliosis predominates organs are T1 hypointense, T2 hyperintense.9 D. Hypoxic-Ischemic Injury - Severe global hypoxic-ischemic injury in this population primarily affects the gray matter structures.10 This predominance of gray matter injury is related to the fact that gray matter contains most of the dendrites where postsynaptic glutamate receptors are located. They are therefore the sites most susceptible to the effects of glutamate excitotoxicity.10 Due to synaptic activity, gray matter is also more metabolically active than white matter. Cerebellar injury can be seen in neonates, but it tends to be more common in older patients.10 The reason for this is not known, but one theory is that the relative immaturity of Purkinje cells (which are normally very sensitive to ischemic damage) in neonates somehow protects the cerebellar cortex.10 References 1. David M. Yousem. Robert D. Zimmerman, and Robert I. Neuroradiology: The Requisites. Grossman Philadelphia, Pa: Mosby, 2010. ISBN 978-0-323-04521-6. 2. v an Gijn J, Kerr RS, Rinkel GJ (2007). “Subarachnoid haemorrhage”. Lancet 369 (9558): 306–18. 3. Rinkel GJ, van Gijn J, Wijdicks EF (1 September 1993). “Subarachnoid hemorrhage without detectable aneurysm. A review of the causes” (PDF). Stroke 24 (9): 1403–9. 4. Warrell, David A; Timothy M. Cox, et al. (2003). Oxford Textbook of Medicine, Fourth Edition, Volume 3. Oxford. pp. 1032–1034. ISBN 0-19-857013-9. 5. Van gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124: 249-78. 6. Brain death: Diagnostic clues on imaging. J Emerg Trauma Shock. 2012 Oct-Dec; 5(4): 372–373. 7. Huang AH. The hot nose sign. Radiology. 2005;235 (1): 216-7. 8. Arii J, Tanabe Y. Leigh syndrome: serial MR imaging and clinical follow-up. AJNR Am J Neuroradiol. 2000;21 (8): 1502-9. 9. Hegde AN, Mohan S, Lath N et-al. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics. 31 (1): 5-30. 10. Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 28 (2): 417-39. VOLUME 11 NO. 1 | 2014