Chapter 5 5.2 Identify the conjugate bases of the following acids: (a
advertisement
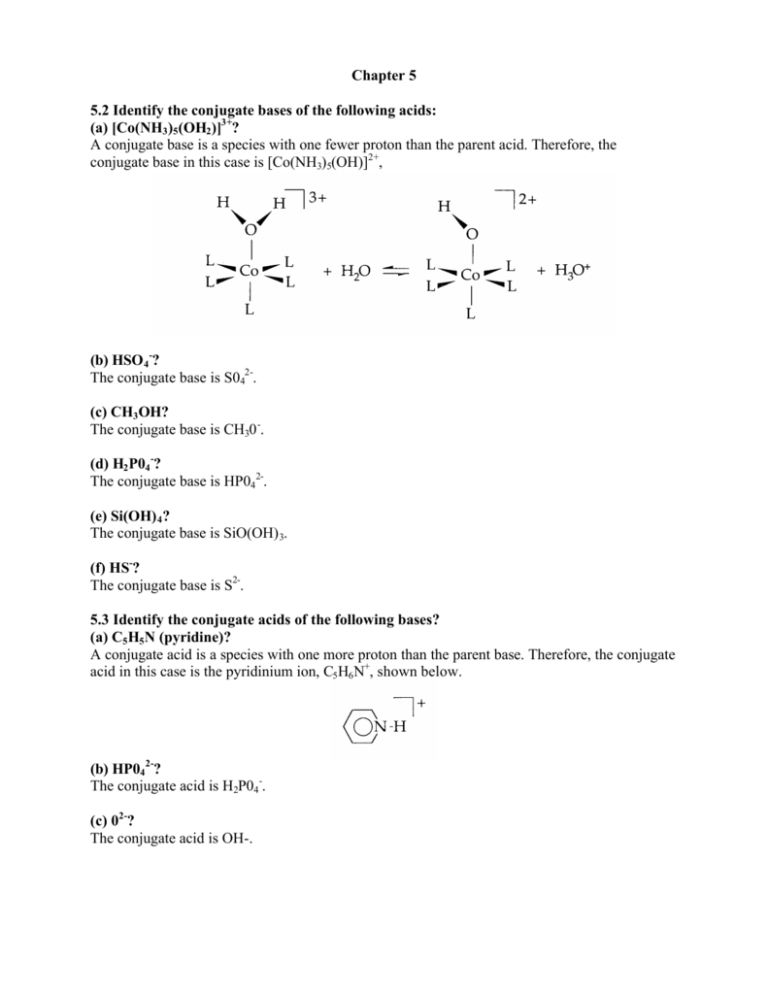
Chapter 5 5.2 Identify the conjugate bases of the following acids: (a) [Co(NH3)5(OH2)]3+? A conjugate base is a species with one fewer proton than the parent acid. Therefore, the conjugate base in this case is [Co(NH3)5(OH)]2+, (b) HSO 4-? The conjugate base is S042-. (c) CH3OH? The conjugate base is CH30 -. (d) H2P04-? The conjugate base is HP042-. (e) Si(OH) 4? The conjugate base is SiO(OH) 3. (f) HS-? The conjugate base is S2-. 5.3 Identify the conjugate acids of the following bases? (a) C5H5N (pyridine)? A conjugate acid is a species with one more proton than the parent base. Therefore, the conjugate acid in this case is the pyridinium ion, C5H6N+, shown below. (b) HP042-? The conjugate acid is H2P04-. (c) 02-? The conjugate acid is OH-. (d) CH3 COOH? The conjugate acid is CH3C(OH) 2+ shown above. (e) [Co(CO)4]? The conjugate acid is HCo(CO) 4, shown below. A drawing of the HCo(CO) 4 molecule, the conjugate acid of the tetrahedral Co(CO) 4- anion. The C atoms of the CO ligands are bound to the Co atom. The O atoms are unshaded. (f) CN-? The conjugate acid is HCN. 5.4 List the bases HS-, F-, I-, and NH2- in order of increasing proton affinity? You should make use of Table 5.1 to answer this question. The species with the greatest proton affinity will be the strongest base, and its conjugate acid will be the weakest acid. The weakest acid will have the smallest value of Ka (or the most positive value of pKa). Since Table 5.1 shows that HI is a stronger acid than HF which is a stronger acid than H2S, a partial order of proton affinity is I- <F- < HS-. Since NH3 is a very weak acid, NH 2- must be a very strong base. Therefore, our final list, in order of increasing proton affinity, is I- <F- < HS - <NH2-. 5.5 Which bases are too strong or too weak to be studied experimentally? (a) C032-, 02-, ClO 4-, and N03- in water? You can interpret the term “studied experimentally” to mean that the base in question exists in water (i.e. it is not completely protonated to its conjugate acid) and that the base in question can be partially protonated (i.e. it is not so weak that the strongest acid possible in water, H30+, will fail to produce a measurable amount of the conjugate acid). Using these criteria, the base C0 32- is of directly measurable base strength, since the equilibrium C032- + H 20 ? HC0 3- + OH - produces measurable amounts of reactants and products. The base 02-, on the other hand, is completely protonated in water to produce OH-, so the oxide ion is too strong to be studied experimentally in water. The bases Cl04- and N0 3- are conjugate bases of very strong acids, which are completely deprotonated in water. Therefore, since it is not possible to protonate either perchlorate or nitrate ion in water, they are too weak to be studied experimentally. (b) HSO 4-, NO 3-, and ClO 4-, in H2SO 4? The hydrogen sulfate ion, HSO 4-, is the strongest base possible in liquid sulfuric acid. However, since acids can protonate it, it is not too strong to be studied experimentally. Nitrate ion is a weaker base than HSO4-, a consequence of the fact that its conjugate acid, HNO 3, is a stronger acid than H 2SO 4. However, nitrate is not so weak that it cannot be protonated in sulfuric acid, so NO3- is of directly measurable base strength in liquid H2SO4. On the other hand, ClO4-, the conjugate base of one of the strongest known acids, is so weak that it cannot be protonated in sulfuric acid, and hence cannot be studied in sulfuric acid. 5.7 Is the pKa for HAsO42- consistent with Pauling’s rules? Pauling’s first rule for predicting the pKa of a mononuclear oxoacid is pKa approximately equals 8 - 5p (where p represents the number of oxo groups attached to the central element). Since p = 1, the predicted value of pKa(1)for H3AsO4is: 8-(5x1)=3. Pauling’s second rule for predicting the pKa of a mononuclear oxoacid is that successive pKa values for polyprotic acids increase by five units for each successive proton transfer. Since pKa(l) for H3AsO4 was predicted to be 3, the predicted value of pKa for HAsO42- which is pKa(3) for H3AsO4, is 3 + (2 x 5) = 13. The actual value, which differs by 1.5 pKa units, is 11.5. This illustrates that Pauling’s rules are only approximate. 5.9 Which of the following is the stronger acid: (a) [Fe(OH2)6]3+ or [Fe(OH2)62+? The Fe(III) complex, [Fe(OH 2)63+, is the stronger acid by virtue of the higher charge. The electrostatic parameter will be considerably higher for z = 3 than for z = 2. The minor decrease in the radius on going from the Fe(II) to the Fe(III) species will enhance the differences in for the two species. (b) [Al(OH2)6]3+ or [Ga(OH2)6]3+? In this case, z is the same but r + d is different. Since the ionic radius, r, is smaller for period 3 Al3+ than for period 4 Ga 3+, r + d for [Al(OH 2)6]3+ is smaller than r + d for [Ga(OH 2)6]3+ and the aluminum-containing species is more acidic. (c) Si(OH) 4 or Ge(OH) 4? As in part (b), above, z is the same but the r + d parameter is different for these two compounds. The comparison here is also between species containing period 3 and period 4 central atoms in the same group, and the species containing the smaller central atom, Si(OH) 4, is more acidic. (d) HClO 3 or HClO 4? These two acids are shown below. According to Pauling’s rule 1 for mononuclear oxoacids, the species with more oxo groups has the lower pKa and is the stronger acid. Thus, HC1O 4 is a stronger acid than HCIO 3. Note that the oxidation state of the central chlorine atom in the stronger acid (+7) is higher than in the weaker acid (+5). (e) H2CrO4 or HMnO 4? As in part (d), above, the oxidation states of these two acids are different, VI for the chromium atom in H 2CrO4 and VII for the manganese atom in HMnO 4. The species with the higher centralatom oxidation state, HMnO4, is the stronger acid. Note that this acid has more oxo groups, three, than H2CrO4, which has two. (f) H3PO 4 or H2SO 4? The oxidation state of sulfur in H2SO4 is VI while the oxidation state of phosphorus in H3PO 4 is only V. Furthermore, sulfuric acid has two oxo groups attached to the central sulfur atom while phosphoric acid has only one oxo group attached to the central phosphorus atom. Therefore, on both counts (which by now you can see are really manifestations of the same thing) H2SO 4 is a stronger acid than H3PO 4. 5.10 Arrange the following oxides in order of increasing basicity? Al2O3, B2O3, BaO, CO 2, Cl2O7, and SO 3? First you pick out the intrinsically acidic oxides, since these will be the least basic. The compounds B2O3, CO 2, C1 207, and SO3 are acidic, since the central element for each of them is found in the acidic region of the periodic table (see the s and p block diagram in the answer to Exercise 5.1 as well as Figure 5.4). The most acidic compound, Cl 2O7, has the highest centralatom oxidation state, +7, while the least acidic, B2O3, has the lowest, +3. Of the remaining compounds, A12O3 is amphoteric, which puts it on the borderline between acidic and basic oxides, and BaO is basic. Therefore, a list of these compounds in order of increasing basicity is Cl2O7 <SO 3 <CO 2 < B 2O3 <A1 2O3 <BaO. 5.18 Identifying acids and bases: (a) SO 3 + H20 à HS04- + H+? The acids in this reaction are the Lewis acids SO3 and H + and the base is the Lewis base OH-. The complex (or adduct) HS04- is formed by the displacement of the proton from the hydroxide ion by the stronger acid SO3. In this way, the water molecule is thought of as an adduct of H and OH-. Since the proton must be bound to a solvent molecule, even though this fact is not explicitly shown in the reaction, the water molecule exhibits Bronsted acidity. Note that it is easy to tell that this is a displacement reaction instead of just a complex formation reaction because, while there is only one base in the reaction, there are two acids. A complex formation reaction only occurs with a single acid and a single base. A double displacement, or metathesis, reaction only occurs with two acids and two bases. (b) Me[B12] + Hg2+ à [B12] + MeHg +? This is a displacement reaction. The Lewis acid Hg2+ displaces the Lewis acid [B12] from the Lewis base CH3. (c) KC1 + SnCl2 à K+ + [SnCl3]-? This is also a displacement reaction. The Lewis acid SnCl 2 displaces the Lewis acid K+ from the Lewis base C1-. (d) AsF3(g) + SbF5(g) à [AsF2][SbF6]? Even though this reaction is the formation of an ionic substance, it is not simply a complex formation reaction. It is a displacement reaction. The very strong Lewis acid SbF5 (one of the strongest known) displaces the Lewis acid [AsF2]+ from the Lewis base F-. (e) EtOH readily dissolves in pyridine? A Lewis acid/base complex formation reaction between EtOH (the acid) and py (the base) produces the adduct EtOH-py, which is held together by the kind of dative bond that you refer to as a hydrogen bond. 5.19 Select the compound with the named characteristic? Strongest Lewis acid: BF3, BCl3, or BBr3? The simple argument that more electronegative substituents lead to a stronger Lewis acid does not work in this case. Boron tribrornide is observed to be the strongest Lewis acid of these three compounds. The shorter boron-halogen bond distances in BF3 and BCl 3 than in BBr 3 are believed to lead to stronger halogen-to-boron p-p π bonding (see Section 5.8). According to this explanation, the acceptor orbital (empty p orbital) on boron is involved to a greater extent in π bonding in BF3 and BC13 than in BBr3, the acidities of BF3 and BC13 are diminished relative to BBr3. BeCl2 or BCl3? Boron trichioride is expected to be the stronger Lewis acid of the two for two reasons. The first reason, which is more obvious, is that the oxidation number of boron in BCl 3 is +3 while for the beryllium atom in BeCl 2 it is only +2. The second reason has to do with structure. The boron atom in BC1 3 is only three-coordinate, leaving a vacant site to which a Lewis base can coordinate. Since BeC1 2 is polymeric, each beryllium atom is four-coordinate, and some Be-Cl bonds must be broken before adduct formation can take place. A piece of the infinite linear chain structure of BeCl 2. Each Be atom is four-coordinate, and each Cl atom is two-coordinate. The polymeric chains are formed by extending this piece to the right and to the left. B(n-Bu) 3 or B(t-Bu)3? The Lewis acid with the unbranched substituents, B(n-Bu) 3, is the stronger of the two because, once the complex is formed, steric repulsions between the substituents and the Lewis base will be less than with the bulky, branched substituents in B(t-Bu) 3. (b) More basic toward BMe 3: NMe 3 or NEt3? These two bases have nearly equal basicities towards the proton in aqueous solution or in the gas phase. Steric repulsions between the substituents on the bases and the proton are negligible, since the proton is very small. However, steric repulsions between the substituents on the bases and molecular Lewis acids like BMe 3 are an important factor in complex stability, and so the smaller Lewis base NMe 3 is the stronger in this case. 2-Me-py or 4-Me-py? As above, steric factors favor complex formation with the smaller of two bases that have nearly equal Brønsted basicities. Therefore, 4-Me-py is the stronger base toward BMe 3, since the methyl substituent in this base cannot affect the strength of the B—N bond by steric repulsions with the methyl substituents on the Lewis acid. 5.21 Choose between the two basic sites in Me 2NPF2? The phosphorus atom in Me 2NPF2 is the softer of the two basic sites, so it will bond more strongly with the softer Lewis acid BH3 (see Table 5.3). The hard nitrogen atom will bond more strongly to the hard Lewis acid BF3. 5.30 Write Brønsted acid-base reactions in liquid HF? (a) CH3CH2OH? Ethanol is a weaker acid than H 2O but a stronger base than H2O due to the electron donating property of the -C2H5 group. The balanced equation is: CH3CH 2OH + HF D H3CH2OH2+ + F(b) NH3? NH3 + HF D NH4+ + F- (c) C6H5COOH? In this case, benzoic acid is a significantly stronger acid than water. Therefore, it will protonate hydrogen fluoride: C6H5COOH + HF D C6H5COO- + H2F +






