SCH4U - Organic Chemistry 1.6 Carboxylic Acids, Esters and Fats
advertisement

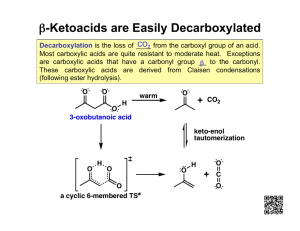
SCH4U - Organic Chemistry 1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, –COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb, and other foods that contain carboxylic acids have a sour, tangy taste. Naming Carboxylic Acids To name organic compounds containing a carboxylic group: 1. Start with the alkane name for the longest chain, including the carbon atom in the carboxyl group 2. Drop the -e suffix of the root compound and replace it with the suffix -oic, followed by the word acid. The simplest aromatic carboxylic acid is benzoic acid, an acid whose common name is the same as its IUPAC name. The sodium salt of benzoic acid, sodium benzoate, is used as a preservative in foods and beverages. Note that, in a compound that combines an alcohol and a carboxylic acid, the alcohol is indicated as a substituent group: hydroxy. Some carboxylic acids have more than one carboxyl group. When naming an acid with two carboxyl groups, use the suffix dicarboxylic acid. SCH4U - Organic Chemistry Citric acid is an example of a compound with three carboxylic groups. Draw the structure of 2-methylbutanoic acid. octanoic acid 3-methylpentanoic acid ethanedioic acid SCH4U - Organic Chemistry Properties of Carboxylic Acids • • • • • • Their molecules are very polar (C=O, O-H) Hydrogen bonding occurs in carboxylic acids with 5 or fewer carbon atoms making them very soluble in water Larger carboxylic acids have decreasing solubility Soluble in polar organic solvents, such as alcohols They affect acid–base indicators React with bases to form ionic compounds and water Esters Many plants naturally produce esters, which are responsible for many of the odours of fruits, flowers, and perfumes. Synthetic esters are often used as flavourings in processed foods, and as scents in cosmetics and perfumes. An ester is characterized by a functional group that is similar to a carboxyl group except that the hydrogen atom is replaced with an alkyl group. The letters R and R in the formula represent alkyl groups in which a carbon atom attaches to the functional group Naming Esters Esters are formed by the condensation reaction of a carboxylic acid and an alcohol. SCH4U - Organic Chemistry When naming esters, follow these steps: 1. Identify the two alkyl groups. 2. Determine which group originated from the carboxylic acid and which originated from the alcohol. 3. Write the name with the alcohol part first and the carboxylic acid part second. Draw the structural formula of ethyl methanoate. methyl hexanoate methyl benzoate SCH4U - Organic Chemistry Properties of Esters Esters are less polar than carboxylic acids and cannot produce hydrogen bonds. • Small esters are soluble in water due to the polarity of their carbon–oxygen bonds • Esters are less soluble in water than carboxylic acids and have lower boiling points • Melting and boiling points of esters are similar to those of the corresponding aldehydes and ketones • Smaller, low–molecular mass esters are gases at room temperature, but the larger, heavier esters are waxy solids Reactions Involving Carboxylic Acids and Esters Formation of Carboxylic Acids Carboxylic acids can be formed by the oxidation of aldehydes in the presence of an oxidizing agent. The roadside Breathalyzer test relies on just such a reaction, in which the oxidizing agent changes colour. Formation of Esters: Esterification Esterification is a condensation reaction in which an alcohol and carboxylic acid react to form an ester and water. Reaction of Esters: Hydrolysis When esters are treated with an acid or a base, the esterification process can be reversed. The ester splits into the carboxylic acid and alcohol components with the addition of a molecule of water. This reaction is called hydrolysis. SCH4U - Organic Chemistry Write equations representing the two chemical reactions that result in the formation of butanoic acid from an alcohol. Draw the structural formula equation representing the reaction that forms propyl butanoate. Also write the names of the compounds involved. Write the structural formula equation for the hydrolysis of ethyl propanoate by sodium hydroxide solution. Draw structural formula equations illustrating the following reactions. Give the IUPAC name for every compound involved. T/I (a) the production of 2-methylpropanoic acid from an aldehyde (b) the production of a carboxylic acid from ethanal (c) the esterification reaction between propanol and hexanoic acid (d) the hydrolysis of methyl butanoate in the presence of sodium hydroxide solution SCH4U - Organic Chemistry Fats and Oils Fats and oils are large ester molecules known as lipids. The long-chain carboxylic acid component is called a fatty acid. The alcohol component is glycerol. Glycerol is a 3-carbon alcohol with three hydroxyl groups, so it can bond with three fatty acids at once. The ester that is formed is called a triglyceride. So, fats and oils are triglycerides. Saponification A triglyceride can be split (hydrolysis) to produce glycerol and the sodium salt of the fatty acid when sodium hydroxide is added to the triglyceride. This resulting sodium salt is commonly called soap, so the reaction is called saponification. SCH4U - Organic Chemistry Structure and Properties of Fats and Oils Insoluble in water The hydrocarbon chains in fatty acids affect the physical state of the lipid. This affects their melting point. Saturated hydrocarbons can “stack” closer to each other increasing their melting point (solids at room temperature). Unsaturated hydrocarbons cannot “stack”, as easily, therefore have lower melting points (liquids at room temperature). Worksheet 1.6: Carboxylic Acids, Esters and Fats p.55 Q. 1, 2, 4, 6, 8, 10