CHM111 Lab – Atomic Emission Spectroscopy – Grading Rubric
advertisement

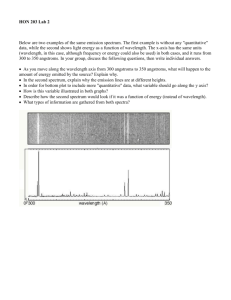
Name_________________________ TeamName______________________ CHM111Lab–AtomicEmissionSpectroscopy–GradingRubric Criteria Pointspossible Pointsearned LabPerformance Printedlabhandoutandrubricwasbroughttolab Safetyandproperwastedisposalproceduresobserved Followedprocedurecorrectlywithoutdependingtoomuch oninstructororlabpartner Workspaceandglasswarewascleanedup 3 2 3 1 1 2 4 1 2 1 20 LabReport Observationsanddatarecordedwithproperunits Correctidentificationofunknowns Calculationsshownclearlyandcompletelywithunits. Question1 Question2(calculationsshownindetailwithunits) Question3(calculationsshownindetailwithunits) Total Subjecttoadditionalpenaltiesaspertheinstructor AtomicEmissionSpectroscopy Introduction Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorptionortheemissionofelectromagneticradiation.Whenelementsorcompoundsareexposedtolargeamounts ofenergyintheformofheat,lightorelectricity,theymayabsorbthisenergy.Whenenergyisabsorbedelectronscan jumpfromtheirgroundstate,orlowestenergylevel,toanexcitedstate,orhigherenergylevel.Electronsinexcited statesareunstableandwilleventuallyreleaseenergyagaintoreturntolowerenergystates.Thisreleaseofenergyis whatweobserveinatomicemissionspectra. Abasicprincipleofquantumtheorystatesthatelectronscanonlyhavecertainspecificenergylevels.Hence, when electrons move from one energy level to another, a specific amount of energy (a quantum) is released or absorbed.Theamountofenergyinanyformofradiationisdirectlyproportionaltoitsfrequency(E=hν),sotheenergy emittedwhenanelectronmovestoalowerenergystatewillhaveadistinctfrequencyandwavelength.Takentogether, all of the wavelengths of light emitted from a particular atom from these electron movements constitute that atom's emissionspectrum.Eachelementorcompoundhasadistinctemissionspectrumthatcanbeusedtohelpidentifyit. Atomicemissionspectracanbethoughtofasatomicfingerprints. Whenahighelectricalpotentialisappliedtoatubeofhydrogengas,theatomswillabsorbsomeoftheenergy and reemit it as light. The distinct wavelengths emitted appear as lines when viewed through a spectroscope. Hydrogenemitslightintheinfrared,visibleandultravioletregions.Thelinesinthevisibleregion,whichcorrespondto electronsdroppingfromhigherenergylevelston=2,areknownastheBalmerseries.Youwillobservethelinesofthe Balmerseriesinthislab.HydrogenalsoemitswavelengthsintheUVregion,knownastheLymanseries,whenelectrons drop to n=1, and in the infrared, known as the Paschen series, when electrons drop ton=3. Theenergylevelsofthe hydrogenatomareschematicallyrepresentedinthediagrambelow. CalculationsInvolvingEnergyLevelsofHydrogen Theenergyoftheelectroninahydrogenatomisgivenbyequation(1)wherenisthequantumnumberofthe energylevel(n=1,2,3,...) (1) Theenergydifferencebetweentwoenergylevelsinhydrogenisgivenbyequation(2)wherenfisthefinalenergylevel oftheelectronandniistheinitialenergyleveloftheelectron.Thiscanbesimplifiedtoequation(3). (2) (3) Whennf>ni,ΔEwillbepositive–energyisabsorbedwhenanelectrongoestoahigherenergylevel.Whennf<ni,ΔE willbenegative–energyisreleasedwhenanelectrondropstoalowerenergylevel.Theenergydifferencebetween levels(ΔE)isequaltotheenergyofthephotonabsorbedoremitted(Ephoton).Theenergyofaphotoniscalculatedwith equation(4)wherePlanck’sconstant(h)=6.626x10-34Js,andthespeedoflight(c)=3.00x108m/s. (4) Oncewemeasurethewavelengthoflightinthehydrogenspectrum,wecanuseequation(4)todeterminetheenergyof thephotons.Sinceweareobservinghydrogen’semissionspectrum,wemustusethenegativeEphotonvalueforΔEin equation(3). (5) WeknowthatfortheBalmerSeries(thevisiblewavelengthsofemittedlightthatwillbeobservedintoday’slab)nf=2. Wecanuseequation(3)tocalculatetheinitialenergylevel(ni)thattheelectrondroppedfrom.InpartBofthe experiment,youwillmeasurethewavelengthsemittedbyhydrogenatomsandultimatelydeterminewhichenergylevel transitionitcorrespondsto. Everyelementhasadistinctspectrumwhichcanbeusedtoidentifyit,muchlikeafingerprint.Heliumwas discoveredwhenscientistslookingatlightfromthesunnoticedanabsorptionspectrumpatternthatdidn’tcorrespond toanyknownelement.InpartAofthethisexperiment,threelampscontaining“unknown"gaseousatomswillbe analyzed.Youwilldeterminetheiridentitybycomparingyourobservedwavelengthvaluestoatableofknownvalues. NotethattheBohrequation(3)onlyappliestohydrogen,sowewillnotcalculateenergylevelsofotherelements. UsefulPhysicalConstantsandConversionFactors Planck'sconstant h=6.626×10−34Js 1angstrom=10─10meter speedoflight c=3.00×108m/s 1nm=10─9meter LaboratoryActivity Equipment handheldspectroscopes,spectroscopes,5000volttransformer,lampscontainingH,He,Hg,andNe. SafetyHazards-DONOTTOUCHTHELAMPORTHEMETALCONNECTIONSWHILETHEAPPARATUSISON! Instructors:Pleasesetupeachapparatusforallthelamps.Placeeachpowersupplyononeofthelabjacksandadjust theheightofthelabjacksothattheslitonthespectroscopelinesupwiththecenterofthelamp.Insertthelamp carefullyinthepowersupplybeingcarefulnottotouchthemiddleofthelamp.Onlyhandletheendsofthelamp. Students: PartA 1.Holdaplastichandheldspectroscopetothefluorescentlightsinthe room,thentosunlightcomingthroughthewindow.Aligntheslitwiththe brightestpartofthelightforthebestresults.Describewhatyouobserve. 2.Turnononeoftheunknownlampsforuptooneminute.Youmay touchthesidesoflamptosteadyitasyoufliptheswitch,butdonot touchthecenterofthelamportheelectricalconnections.Afterone minute,turnthelaboffandallowittorestforatleastoneminutebefore turningitonagainifnecessary. 3.Lookthroughthespectroscopesinfrontofthelampsforunknowns#1, #2,and#3.Recordthewavelengthsofthemajorlinestotwosignificant figures.Notetheunitonthespectroscope.Forsomeoftheunknowns, youwillseeanalmostcontinuousspectrumratherthandiscretelines. Recordtherangeandcenterwavelengthofthisbroadbandofcolor. ComparethewavelengthstothoseinTable1anddeterminetheidentity ofeachunknown. PartB 3.UsethesametechniqueasinPartAtorecordthecolorandwavelengthsofthehydrogenspectrum.Youshouldbe abletoseethreeorfourlines.UsethesewavelengthstocalculatetheΔEforthetransitionandthentheinitialenergy leveloftheelectronforeachtransition(ni)Rememberthatnf=2forthevisibleregion(theBalmerseries). AtomicEmissionSpectroscopy:DataSheetName_____________________________ PartA: 1.Recordobservations: fluorescentlight sunlight Basedonwhatyouobserved,describeisthedifferencebetweenacontinuousspectrumandalinespectrum. 2.Recordcolorandwavelengthofspectrallinesfortheunknowns.Includethecorrectunitswiththewavelength. Unknown#1 Unknown#2 Unknown#3 Color Wavelength Color Wavelength Color Wavelength Usethefollowingtabletodentifyeachoftheunknownelements: CHARACTERISTICSPECTRALLINES* He(nm) Hg(nm) Ne(nm) 447 405 540 502 436 583 588 546 585 668 579 640 707 Unknown#1__________________Unknown#2__________________Unknown#3__________________ *Thisisanabbreviatedtable,butitshowssufficientwavelengthsforidentifyingtheunknownelements.Longertables areintheHANDBOOKOFCHEMISTRYandPHYSICS ReportPage1of3 AtomicEmissionSpectroscopy:PostLabName_____________________________ PartB: 1.Recordcolorandwavelengthsforthehydrogenspectrum,thenuseequations(4),(5)and(3)tocalculateΔEandni. Color wavelength Ephoton nf ni(makeΔEnegative,useseparatepageifneeded) 2 2 2 2 ReportPage3of3 AtomicEmissionSpectroscopy:PostLabName_____________________________ 1.Rankthefollowingradiationsfromshortesttolongestwavelength. Radiowaves Infraredwaves Gammarays microwaves Xrays Shortestλ____________________________________________________________longestλ 2.WhichofthefollowingelectronswillemitlightofLONGERWAVELENGTH? Anelectrondroppingfromn=3ton=2ORanelectrondroppingfromn=4ton=3?Calculatethewavelengthfor eachtransitiontojustifyyouranswer. 3.Theionizationenergyistheenergyneededtoremoveanelectronfromanatomwhichcorrespondstoaraisingthe electronfromn=1toanorbitthathasn=∞.Whatistheenergyneededtoremovetheelectronfromahydrogenatom? ReportPage3of3