1 - Holland Public Schools
advertisement

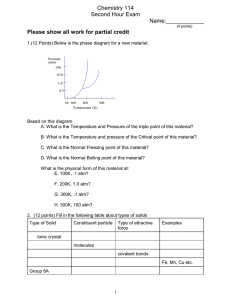
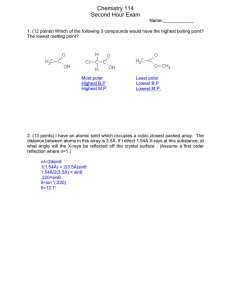
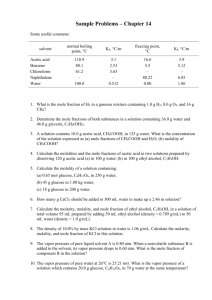
UNIT VIII – SOLUTIONS REVIEW I. Good things to know: Mixtures: homogeneous and heterogeneous Types of colloids: aerosol, foam, emulsion, sol types of solutions solution terms: solute, solvent, insoluble, soluble molecular view of solution process saturated, unsaturated, supersaturated; fractional crystallization solubility of solid solutes vs. temperature gas solubility vs. temperature and pressure (Henry’s Law) colligative properties, van’t Hoff Factor II. Essays/Short Answer 1. Explain why ethanol (CH3CH2OH) is soluble in both water and carbon tetrachloride. 2. Explain why in a dilute solution, molarity and molality will have the same value. 3. Give the estimated van’t Hoff factor for the following substances: ______ a. NaCl ______ b. CH3CH2OH (ethanol) _____ c. Ag2SO4 III. Problems 1. A solution is made by dissolving 49g of NaCl in enough water to make 1.00L of solution. Assume that the density of the solution is 1.00 g/cm3. Calculate the mass percent, molarity, molality, and mole fraction of NaCl. UNIT VIII – SOLUTIONS REVIEW 2. Using the chart shown o a. what is the solubility of: HCl at 30 C? b. from the examples below, which is the most o soluble at 20 C. NH3 KNO3 NaCl NaNO3 c. Explain why the solubility of HCl, NH3, and SO2 decrease as temperature increases. d. If you have 25g of KNO3, how much water would you need to make a saturated solution at room temperature (25oC) ? o e. If you added 50.0g of KCl to 10g of water at 40 C, would the solution be saturated, supersaturated, or unsaturated? If it is saturated, how much is left over? 3. Pentane (C5H12) and hexane (C6H14) form an ideal solution. At 25oC the vapor pressures of pentane and hexane are 511 and 15θ Torr, respectively. A solution is prepared by mixing 25 mL pentane (density = 0.63 g/mL) with 45 mL hexane (density = 0.66 g/mL). What is the total vapor pressure of the resulting mixture of gases? UNIT VIII – SOLUTIONS REVIEW 4. A solution of sodium chloride in water has a vapor pressure of 21.9 Torr at 25 oC. What is the mole fraction of NaCl in this solution? What would be the vapor pressure of this solution at 45 oC? The vapor pressure of pure water is 23.8 Torr at 25 oC and 71.9 Torr at 45oC. 5. You form a solution by dissolving 50.00g of the nonelectrolyte benzoic acid (C7H6O2) in 200.0 mL of carbon tetrachloride (CCl4) (D = 1.594 g/mL). Using the table on the bottom of p. 8 for any reference you need: a. calculate the molality of the solution b. calculate the boiling point of the solution c. calculate the freezing point of the solution 6. An aqueous solution is created by dissolving 25.03g of iron(III) chloride (FeCl 3) (i=3.4) in 300.0 mL of water. Calculate the boiling and melting points of the solutions. UNIT VIII – SOLUTIONS REVIEW 7. The molecular weight and formula of a hydrocarbon are to be determined through the use of the freezing point depression method. The hydrocarbon is known to be 86% carbon and 14% hydrogen by mass. In the experiment, 3.72g of the unknown hydrocarbon were placed into 50.0g of liquid benzene (C6H6). The freezing point of the solution was measured to be 0.06oC. The normal freezing point of benzene is 5.50oC, and the freezing point depression constant for benzene is 5.12 oC/m. a. What is the molecular weight of the compound? b. What is the molecular formula of the hydrocarbon? c. What is the mole fraction of benzene in the solution? d. If the density of the solution is 875 g/L, what is the molarity of the solution?