Chemistry 114 Second Hour Exam
advertisement

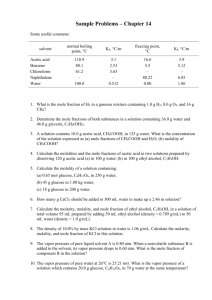
Chemistry 114 Second Hour Exam Name:____________ 1. (8 points) Identify the dominant intermolecular force present in particles of the following solids NH4Cl C2H6 NH3 N2 2. (12 points) In each of the following groups of substances pick the one that has the given property. Justify your answer Highest boiling point: N2 , O2, NO Lowest Freezing point H2O, NaCl, HF Strongest hydrogen bonding: NH3, PH3, SbH3 Greatest viscosity: CH3CH2CH2CH3, CH3CH2OH, HOCH2CH2OH 1 3. (10 points) In the Germanium crystal there is a 3.265 D distance between atoms. What angle will the first order (n=1) diffraction line be for 1.54 D wavelength X-rays 4. (10 points) What types of solids will each of the following substances form CO Ru KBr C P4 5. (10 points) The vapor pressure of water is 525.8 torr at 90oC and 760 torr at 100oC. What is the )Hvap for water? 2 6. (10 points) I have a substance that at 1 atm of pressure melts at -20oC and boils at 40oC. When you have the same substance at 0.1 atm the material sublimes at -10oC. Make a reasonable phase diagram for this compound. On your phase diagram note the location of the triple point and the critical point. 7. (10 points) I have a solution that is 5% by weight acetic acid. What is the molality of this solution? (MW acetic acid = 60.052) 8. (10 points) What is the boiling point of the 5% acetic acid solution that you calculated molality for in problem 7? (Kb water = 0.51 oC@kg/mol) 3 9. A (5 points) The vapor pressure of dimethyl ether (CH3OCH3) is 623 tor at 300oC. The vapor pressure of methanol is 18.7 tor at this same temperature. If I mix 1 mole of dimethyl ether with 3 moles of methanol, what is the vapor pressure of a mixture? B(5 points) Say I noticed that the above solution got hotter when I mixed it together, would you expect your answer in A to be higher or lower? Why? 10. (10 points)What is a colloid? 4