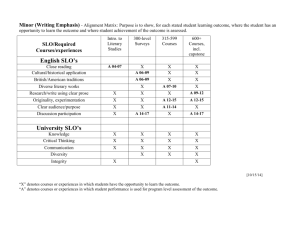
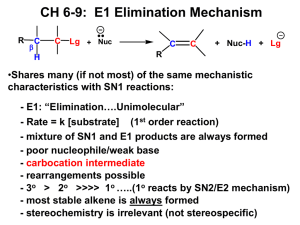
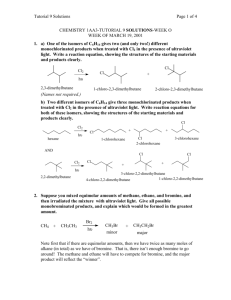
Chapter 9 Supplemental Problems
advertisement

Chapter 9 Supplemental Problems Chemistry 12A Fall 2011 1) What product would you expect to obtain from an SN2 reaction of OH- with (R)2-bromobutane? Show the stereochemistry of both reactant and product. (11.2) 2) Which substance in each of the following pairs is more reactive as a nucleophile? (11.5) a. (CH3)2N- or (CH3)2NH b. (CH3)3B or (CH3)3N c. H2O or H2S 3) Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)3CCl, (CH3)2CHCl 4) What product(s) would you expect from the reaction of (S)-3-chloromethyloctane with acetic acid? Show the stereochemistry of both reactant and product. (11.8) 5) Assign configuration to the following substrate and show the stereochemistry and identity of the product you would obtain by SN1 reaction with water. Red = Br (11.10) 6) Rank the following substances in order of their expected SN1 reactivity: CH3CH2Br, H2C=CHCH(Br)CH3, H2C=CHBr, CH3CH(Br)CH3 (11.11) 7) 3-bromo-1-butene and 1-bromo-2-butene undergo SN1 reactions at nearly the same rate even though one is a secondary halide and the other is primary. Explain. (11.12) 8) Predict whether each of the following substitution reactions is likely to be SN1 or SN1: (11.14) 9) Which two products would you expect from the following reaction? Identify the minor and major products and explain why you chose each. (Practice 11.3) 10) What alkyl halides might the following alkenes have been made from? (11.16) 11) What stereochemistry do you expect for the alkene obtained by E2 elimination of (1R, 2R)-1,2-dibromo-1,2-diphenylethane? (11.17) 12) Tell whether each of the following products are likely to be SN1, SN2, E1, or E2. (Practice 11.5)