sol graph assign - Norbraten
advertisement
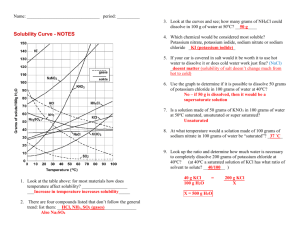
SCH 3U Solubility Graphs Define the following terms in your own words and provide an example for each; Term Solute Definition Example Solvent Solution Unsaturated solution Saturated solution Super saturated solution Use the solubility curve graph provided to answer the following questions. 1. What are the customary units of solubility on solubility curves? ___________ 2. Define solubility. __________________________________________________ 3. According to the graph, the solubility of any substance changes as _________ changes. 4. List the substances whose solubility decreases as temperature increases. ___________ _______________________________________________________________ 5. Which substance is least affected by temperature changes? ____________________ 6. How many grams of ammonium chloride (NH4Cl) at 50°C? ______________________ 7. _____________ and ____________ have the same solubility at approximately 78°C. 8. Which compound is least soluble in water at 10°C? ___________ 9. How many grams of KNO3 can be dissolved at 50°C? __________ 10. Are the following solutions unsaturated, saturated, or supersaturated? a. 45g of NaNO3 in 100 g of water at 30°C.____________ b. 60g of KClO3 in 100 g of water at 60°C.____________ 11. How many grams of sodium chloride, NaCl are required to saturate 100 grams of water at 100° C? ______ 12. How many grams of NaNO3 are required to saturate 100 grams of water at 90°C?____ 13. How many grams of KI will saturate water at 20°C?________ 14. At what temperature would 25g of potassium chlorate (KClO3) dissolve? _______ 15. At what temperature would 55g of NH4Cl dissolve? ________ 16. 89 g NaNO3 is prepared at 30°C. a) Will all of the salt dissolve? ____________ b) What mass of NaNO3 will dissolve at this temperature? __________ 17. If 25 grams of NH4Cl is dissolved at 50°C, how many additional grams NH4Cl would be needed to make the solution saturated at 80°C? _________ 18. At 50°C, how many grams of KNO3 will dissolve? _________ 19. At 70°C, how many grams of cerium (III) sulfate (Ce2(SO4)3) dissolve? __________ 20. Determine if each of the following is unsaturated, saturated, or supersaturated. a. 55g of NH3 at 20°C _________ f. 80g of NaNO3 at 10°C _________ b. 10g of Ce2(SO4)3 at 10°C _________ g. 145g of NaNO3 at 80°C _________ c. 125g of KNO3 at 60°C _________ h. 35g of NaCl at 100°C d. 65g of NH4Cl at 80°C _________ e. 12g of NH3 at 90°C _________ _________ Use the following solubility graph to answer the following questions. 1. What mass of potassium chlorate can be dissolved in 100 mL at 70 °C? __________________ 2. What is the solubility of ammonium chloride at 50 °C? ___________ 3. From the graph, determine the temperature of potassium chlorate when its solubility is: 20 g / 100 mL ______ 50 g / 100 mL ______ 4. At what temperature are the solubilities of potassium nitrate and potassium chloride approximately equal? _________ 5. Determine the mass of potassium nitrate that will come out of solution when the temperature is decreased from 50 °C to 20 °C? __________________ 6. Write a general statement concerning the solubility of the ionic substances as the temperature increases. _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ Creating Solubility Graphs On the graph paper provided, plot graphs of solubility (dependent axis (y)) versus temperature for the following chemicals. Orient your graph in landscape and use both the left side for ammonium chloride and a new axis on the right for carbon dioxide, plot both curves on the same graph. Ammonium Chloride Solubility (g/L) Temperature °C 300 0.0 372 20.0 458 40.0 550 60.0 658 80.0 771 100.0 Carbon dioxide Solubility (g/L) Temperature °C 3.4 0.0 1.8 20.0 1.2 40.0 0.86 60.0 Questions 1. From the graph, determine the solubility of ammonium chloride at: i. 10°C _____ ii. 30 °C _____ iii. 70 °C _____ 2. From the graph, determine the solubility of carbon dioxide at: i. 10°C _____ ii. 30 °C _____ iii. 50 °C _____ 3. From the graph, determine the temperature of ammonium chloride when the solubility is i. 400 g/L _____ ii. 650 g/L _____ 4. From the graph, determine the temperature of carbon dioxide when the solubility is i. 1.6 g/L _____ ii. 2.4 g/L _____ 5. Make a general statement concerning the solubility of each of the substances as the temperature increases. ___________________________________________________________________ ___________________________________________________________________ __________________________________________________________________ 6. For each of the following temperature changes, determine the mass of ammonium chloride that would have to be added to keep the solution saturated: i. 0 °C to 20°C _____ ii. 40°C to 100°C ______ iii. 10°C to 50°C _____ 7. Determine the mass of carbon dioxide that will come out of solution, when the temperature is increased in each of the following situations: i. 0°C to 60°C _____ ii. 10°C to 50°C _____ 8. Determine the mass of ammonium chloride that will come out of solution when the temperature is decreased from 100°C to 10°C. _____ Textbook work: Read pgs. 312-316. Answer practice questions on ****pg. 316 # 1,2 SOLUBILITY CURVE WORKSHEET KEY Use your solubility curve graphs provided to answer the following questions. 1. What are the customary units of solubility on solubility curves? Degress Celsius and grams of solute/100g of water 2. Define solubility. A measure of how much solute can dissolve in a given amount of solvent. 3. According to the graph, the solubility of any substance changes as temperature changes. 4. List the substances whose solubility decreases as temperature increases. NH3 and Ce2(SO4)2 5. Which substance is least affected by temperature changes? NaCl, 6. How many grams of ammonium chloride (NH4Cl) at 50°C? 50g 7. NaCl and KClO3 have the same solubility at approximately 78oC. 8. Which compound is least soluble in water at 10°C? KClO3 9. How many grams of KNO3 can be dissolved at 50oC? 80g 10. Are the following solutions unsaturated, saturated, or supersaturated? a. 45g of NaNO3 in 100 g of water at 30°C. saturated b. 60g of KClO3 in 100 g of water at 90°C. supersaturated 11. How many grams of sodium chloride, NaCl are required to saturate 100 grams of water at 100° C? 40g 12. How many grams of NaNO3 are required to saturate 100 grams of water at 75°C? 140g 13. How many grams of KI will saturate water at 20°C? 33g 14. At what temperature would 25g of potassium chlorate (KClO 3) dissolve? 600C 15. At what temperature would 60g of NH4Cl dissolve? 700C 16. 89 g NaNO3 is prepared at 30°C. a) Will all of the salt dissolve? No_ b) What mass of NaNO3 will dissolve at this temperature? 95g 17. If 50 grams of NH4Cl is dissolved at 50°C, how many additional grams NH4Cl would be needed to make the solution saturated at 80°C? 15g 18. At 50°C, how many grams of KNO3 will dissolve? 80g 19. At 70°C, how many grams of cerium (III) sulfate (Ce2(SO4)3) dissolve? 5g 20. Determine if each of the following is unsaturated, saturated, or supersaturated. a. 55g of NH3 at 20oC supersaturated f. 78g of NaNO3 at 10oC. saturated b. 10g of Ce2(SO4)3 at 10oC unsaturated g. 145g of NaNO3 at 80oC. saturated c. 110g of KNO3 at 60oC. supersaturated h. 35g of NaCl at 100oC. unsaturated d. 65g of NH4Cl at 80oC. saturated e. 12g of NH3 at 90oC. supersaturated