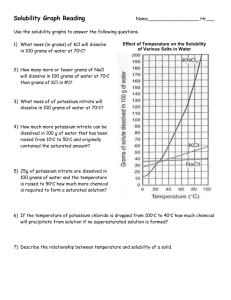
Solubility Curve
advertisement
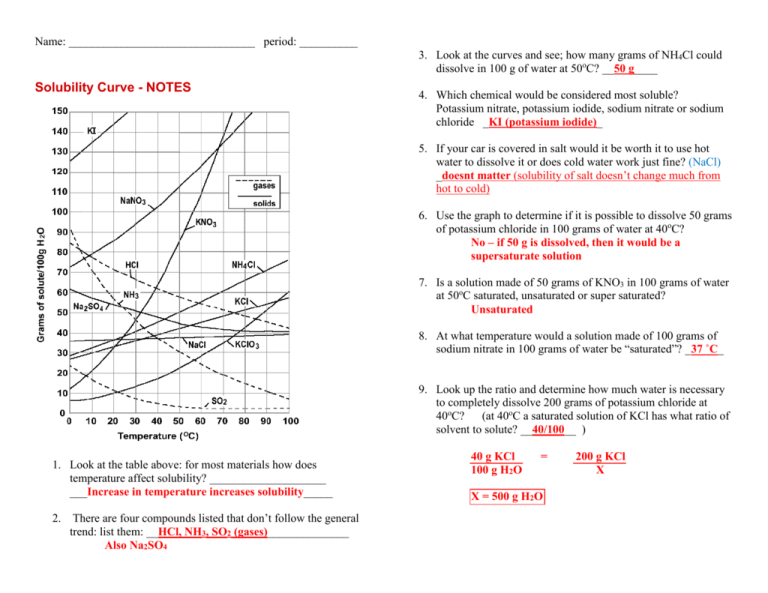
Name: ________________________________ period: __________ 3. Look at the curves and see; how many grams of NH4Cl could dissolve in 100 g of water at 50oC? __50 g____ Solubility Curve - NOTES 4. Which chemical would be considered most soluble? Potassium nitrate, potassium iodide, sodium nitrate or sodium chloride _KI (potassium iodide)_ 5. If your car is covered in salt would it be worth it to use hot water to dissolve it or does cold water work just fine? (NaCl) _doesnt matter (solubility of salt doesn’t change much from hot to cold) 6. Use the graph to determine if it is possible to dissolve 50 grams of potassium chloride in 100 grams of water at 40oC? No – if 50 g is dissolved, then it would be a supersaturate solution 7. Is a solution made of 50 grams of KNO3 in 100 grams of water at 50oC saturated, unsaturated or super saturated? Unsaturated 8. At what temperature would a solution made of 100 grams of sodium nitrate in 100 grams of water be “saturated”? _37 ˚C_ 9. Look up the ratio and determine how much water is necessary to completely dissolve 200 grams of potassium chloride at 40oC? (at 40oC a saturated solution of KCl has what ratio of solvent to solute? __40/100__ ) 1. Look at the table above: for most materials how does temperature affect solubility? ____________________ ___Increase in temperature increases solubility_____ 2. There are four compounds listed that don’t follow the general trend: list them: __HCl, NH3, SO2 (gases)______________ Also Na2SO4 40 g KCl 100 g H2O = X = 500 g H2O 200 g KCl X