Solubility Quiz
advertisement

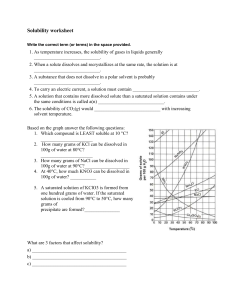
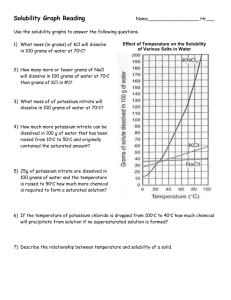
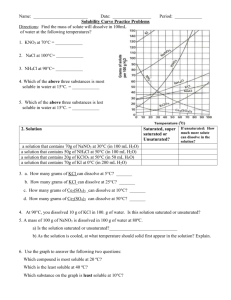
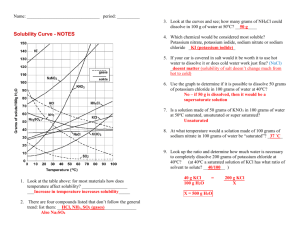
Solubility Quiz 1) What chemical (name or formula) was used to be dissolved in the Solubility lab? 2) Make a statement about the ability of the substance to be dissolved and the temperature of the water? 3) What are the two parts of any solution called? 4) Describe the condition of “slightly soluble”. 5) How might you dissolve crystals of Copper Sulfate in water? 6) Looking at the overhead, answer the following questions – a) What is the amount of KCl dissolved at 50 degrees? b) What is the temperature of the KNO3 for dissolving 150 grams? c) What is the amount of solid left in the container when you try and dissolve 55 grams of NaCl at 20 degrees? 7) Name the three conditions that a solution can have as you dissolve material into “it”? 8) If you put 30 grams of solid into 70 grams of water, what will be the percentage of concentration? 9)A 55 grams sample of 20% sugar water is presented to you. What will be the amount of sugar and the amount of water for each. Answer in grams 10)From the drawing models show how would you make the solution more concentrated and how do you make it more dilute?