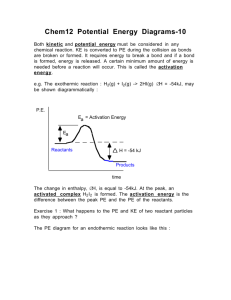
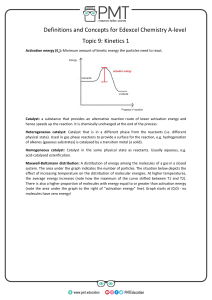
Name ................................................ Homework Rates 2 Date due
advertisement
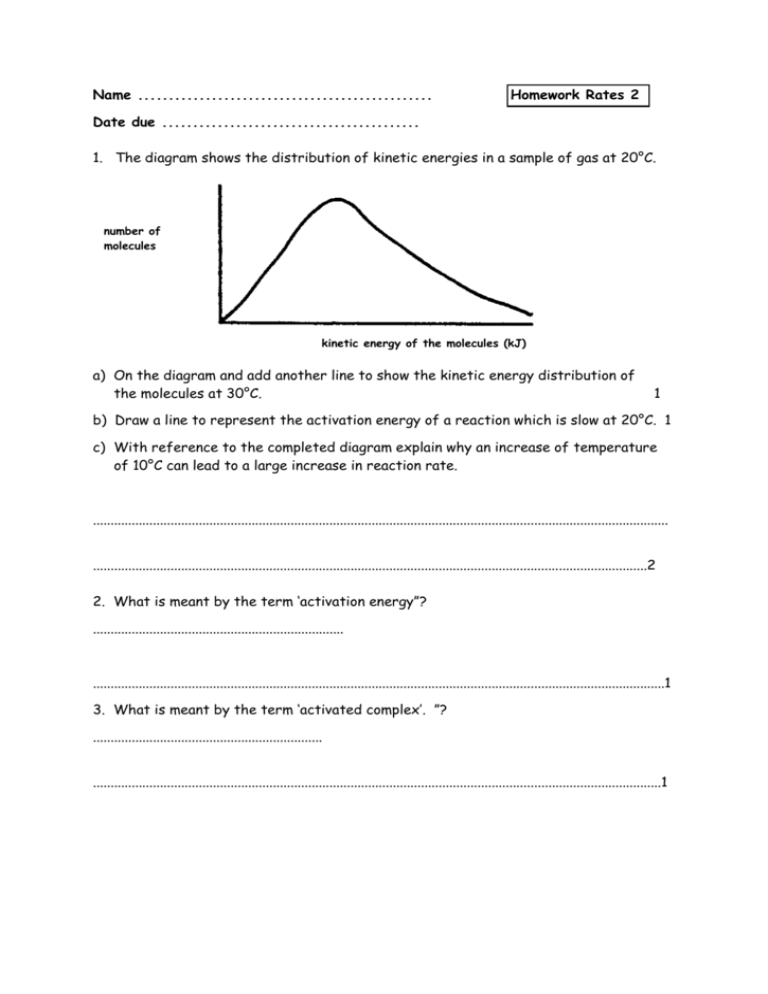
Name ................................................ Homework Rates 2 Date due .......................................... 1. The diagram shows the distribution of kinetic energies in a sample of gas at 20°C. number of molecules kinetic energy of the molecules (kJ) a) On the diagram and add another line to show the kinetic energy distribution of the molecules at 30°C. 1 b) Draw a line to represent the activation energy of a reaction which is slow at 20°C. 1 c) With reference to the completed diagram explain why an increase of temperature of 10°C can lead to a large increase in reaction rate. ................................................................................................................................................................... .............................................................................................................................................................2 2. What is meant by the term ‘activation energy”? ....................................................................... ..................................................................................................................................................................1 3. What is meant by the term ‘activated complex’. ”? ................................................................. .................................................................................................................................................................1 4. Potential energy (kJ) a)i) Is this an endothermic or an exothermic reaction? ............................................................. 1 ii) Explain your answer. ................................................................................................................. ......................................................................................................................................................... 1 b) What is the value of i) the activation energy for the forward reaction? .........................................................1 ii) the enthalpy change for the reaction? ..........................................................1 iii) the energy of the activated complex? ........................................................... 1 c) The reaction shown can be speeded up by the use of a suitable catalyst. What effect does a catalyst have on i) the enthalpy change for the reaction? ............................................................ 1 ii) the activation energy for the forward reaction.............................................................1 5. 2HI(g) H2(g) + I2(g) The reaction above is reversible. The activation energy for the forward reaction is 80 kJ and the reverse reaction is 50 kJ. a) On the graph paper shown below how the potential energy varies as the reaction proceeds. 160 140 Potential energy (kJ) 120 100 80 60 reactants 40 20 0 reaction pathway b) Is the forward reaction is exothermic or endothermic.........................................1 c) Gold and platinum both catalyse the reaction. For the forward reaction EA using gold is 50 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the graph. 2 ii) which is the better catalyst for the reaction? Explain your choice. ................................................................................................................................... 2 d) The gold and platinum catalysts are used in the solid state. Are the catalyst heterogeneous or homogeneous catalysts? Explain your choice. ......................... .........................................................................................................................................2 Total = 20 marks