sbs-017 basic biochemistry - Personal Webspace for QMUL

SBS-017 BASIC BIOCHEMISTRY
COURSES ORGANISER
DR R.W. JANES (ROOM G07 – Joseph Priestley Building)
COURSE LECTURERS: DR R.W. JANES
PROF. R.W. PICKERSGILL
DR. J. SULLIVAN
MR. J. PUDDEFOOT
On the following pages a detailed course synopsis lists the objectives and topics to be covered for each of the 22 lectures on this course.
PRACTICALS
:
The practical sessions for this course will be allocated at a later time and you will be informed when they are for you by individual timetable.
ABSENCES:
Attendance at lectures “may” be monitored by FIVE UNANNOUNCED mini-tests . If you miss a practical/piece of coursework you will have earned no marks and the default is that zero is recorded on the mark sheet. If you miss a practical/coursework for good reason, you should download and complete the form EC1 (from the SBCS website) and submit this to
Reception in the Fogg building within a week. If you are absent for more than five consecutive days then a medical (or other) certificate is required. In genuine cases the first practical missed in each semester will be awarded a mark which is the mean of the marks for other coursework on that module. The second piece of coursework that is missed for good reason will be awarded a maximum of 40%. All other missed coursework will earn no marks.
Thus in each semester only two such compensatory marks in total will be awarded across all your modules, and only where the coursework item is 5% or less of the total module credit.
You are not allowed to miss a practical and submit a report based on another student’s results without the agreement of the module organiser (otherwise it is plagiarism). Also, you are not allowed to copy some part or all of another student’s report and present it as your own work
(this is also plagiarism).
For students with serious medical, or other, problems leading to extensive absence(s) you need to record that there is an issue on form EC2 (form on the web site) without giving any details. You then must contact your Adviser or one of the Senior Academic Advisers and after that we will consider how your studies will best be advanced.
EXAMINATION:
The exam will be 2 hours 15 minutes long. There will be one compulsory multiple-choice question in Section A, and two further Sections, B and C, comprising of two short answer questions in each. You will be required to answer ONE from Section B and ONE from Section
C.
TEXTBOOK:
The recommended text is Berg, Tymoczko & Stryer, Biochemistry ( 6TH EDITION )
W.H.Freeman & Company (2006). This text will be referred to throughout the course, and all overheads used will be taken from this text. For summaries of the lectures (displayed one week in advance) you should go to the following Web site: http://webspace.qmul.ac.uk/rwjanes/basbio.htm
If you wish to contact me by far the best method is to E-MAIL me on r.w.janes@qmul.ac.uk
COURSE SYNOPSIS
DR R.W.JANES
(LECTURES 1-8)
LECTURE 1
Amino Acids
Objectives: To establish that 20 amino acids are used in the proteins of living organisms; to explain their nomenclature; to explain how their different properties place them into different environments in proteins.
20 'common' amino acids used in proteins.
The CORN Law denotes L isomer amino acid.
5 key features of an amino acid:
Size Shape Charge Hydrogen bonding capacity Chemical reactivity.
Cysteine can form disulphide bonds known as cystine.
LECTURE 2
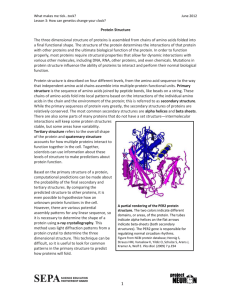
The Principal Aspects of Protein Structure
Objectives: To emphasise that amino acids join to form polypeptide chains (proteins), and how the sequences of these chains, the Primary Structure, are unique to any given protein. To reveal how these chains can form distinct structural features within a protein, the Secondary
Structure, including helices and sheet-like structures.
The peptide bond is planar and usually trans in conformation.
A chain of amino acids is read N- (amino) terminal to C- (acid 'carboxyl') terminal.
A polypeptide chain is the primary structure of a protein and unique to that protein.
There are specific periodic structural features which polypeptide chains can adopt.
These are the secondary structure features of a protein.
Major ones:
helix,
sheet (anti- & parallel) and
turn.
Others: 3
10
helix,
helix & collagen helix.
Helices differ in rise per residue & pitch.
sheets: antiparallel more stable than parallel.
LECTURE 3
The Principal Aspects of Protein Structure II
Objectives: To provide awareness of higher levels of protein structure, (Tertiary and
Quaternary Structure), and introduce the principal aspects of protein conformation and folding.
Tertiary structure is the conformation of a single polypeptide chain.
The primary structure determines the tertiary structure.
Quaternary structure is the conformation adopted by proteins containing more than one polypeptide chain.
Protein conformation buries hydrophobic residues forming the protein core.
Proteins fold via the formation of stable partially correct secondary structure features within the intermediate stages.
LECTURE 4
Protein Modification and Protein Cleavage
Objectives: To realise the importance of how and why proteins are post-translationally modified both by changes to specific amino acids, or by selective cleavages between amino acids. To illustrate these concepts using two worked examples.
Acetylation of the N-terminal of a protein.
Formation of Hydroxyproline required for collagen.
Features of protein glycosylation.
Features of protein phosphorylation.
Transportation of a protein in a precursor form prior to the final structure.
LECTURE 5
Protein purification techniques I
Objectives: To establish that a wide variety of physical properties of proteins can be employed in procedures used for their purification. To describe the principals behind particular purification techniques.
Protein isolation methods.
Proteins need to be stabilised.
Protein solubilities change with:
Salt concentration Organic solvents pH.
Proteins can be separated by crystallising them.
Changes to protein solubility crucial to protein crystallography.
LECTURE 6
Protein purification techniques II
Objectives: To provide details of, and principals behind, the major techniques used in protein purification. To equip students with a fundamental awareness of laboratory procedures.
Chromatographic separation techniques:
Ion exchange Gel filtration Affinity others.
Electrophoresis:
Gel SDS-page Isoelectric focusing.
Ultracentrifugation:
Sedimentation Preparative ultracentrifugation.
LECTURE 7
Methods of Studying Protein Structure I
Objectives: This, (and lecture 8) are designed to create awareness that one essential feature of modern biochemistry are the advantages that can be gained from the knowledge of a proteins three-dimensional conformation. Here this is through the solution technique of NMR.
Nuclear Magnetic Resonance (NMR):
- Proteins contain hydrogen atoms.
- Their nuclei can act as 'bar magnets'.
- Magnets in close proximity interact.
- Hence measuring the extent of interaction allows for structure determination.
LECTURE 8
Methods of Studying Protein Structure II
Objectives: To outline a further technique for gaining the knowledge of a proteins three-dimensional conformation, here X-ray crystallography. Also, how circular dichroism can be used to assist in providing invaluable information about a protein structure.
X-ray Crystallography:
- Proteins in crystal form scatter X-rays.
- From the scattering patterns, reconstruction can be undertaken of the structure of the protein that gave rise to the particular pattern recorded.
- Hence a protein structure can be produced.
Circular Dichroism (CD):
- Focuses on how proteins interact with circularly polarized light.
- Different secondary structure features interact in distinctly different ways.
- Can determine the percentage present in a protein of each type of secondary structure.
DR J. SULLIVAN (LECTURES 9-11)
LECTURE 9
Enzymes; basic principles
Objectives: To introduce the basic concepts and definitions of enzymology .
Enzymes are catalysts
Enzyme specificity
Enzymes activity is regulated
Enzymes interconvert forms of energy
Enzymes stabilise the transition state
LECTURE 10
Enzyme kinetics
Objectives: To provide an introduction to the kinetic analysis of enzyme activity .
Time course of an enzyme catalysed reaction
Michaelis-Menten kinetics
Meaning of k cat
, K
M
and V max
Kinetic perfection-k cat
/K
M
Enzyme inhibition
LECTURE 11
Enzyme catalysis
Objectives: To describe formation of the enzyme-substrate complex and to illustrate the importance of this intermediate in enzyme catalysis .
The active site concept
Enzyme-substrate complementarity
Fischer-lock and key hypothesis
Koshland-induced fit hypothesis
Enzyme-substrate interactions
PROF R.W. PICKERSGILL (LECTURES 12-15)
LECTURE 12
Enzyme case study-Chymotrypsin.
Objectives: To expand on the concepts introduced in the previous three lectures through a detailed study of a named example.
Chymotrypsin is a serine protease
Formation of a covalently bound intermediate
The catalytic triad
Transient tetrahedral intermediate
Comparison with trypsin and elastase
LECTURE 13
Allostery
Objectives: To introduce the concept of allostery as an explanation for the observation of sigmoidal binding curves.
Sigmoidal binding curves
Models of allostery (WMC, etc)
Haemoglobin as an example of an allosteric protein
Allostery in enzymes-aspartate transcarbamylase
LECTURE 14
Ion transport
Objectives: To introduce the basic concepts of transmembrane solute transport, with particular reference to ion transport. To relate these processes to nerve impulse transmission .
Channels-introduction
Ion channels-the acetylcholine receptor channel
Voltage gated channels
Nerve impulses and synapses
LECTURE 15
Ion pumps and other transport proteins
Objectives: To expand on the concepts introduced in Lecture 19 to include coupled transport of more than one solute and active transport driven by ATP hydrolysis and light .
Ion pumps-introduction
Active transport-Na
+
/K
+
ATPase
Metabolite transport-Lactose permease
Light driven transport-bacteriorhodopsin
DR. R.W. JANES (LECTURES 16-18)
LECTURE 16
Metabolic Energy: Generation and storage
Objectives: To illustrate how metabolic pathways work, and are linked together by various soluble cofactors to transport energy and chemical groups.
How a thermodynamically favourable reaction can drive a thermodynamically unfavourable one.
ATP as a free energy donor in most energy-requiring processes.
NADH and NADPH as electron donors.
Coenzyme A as a universal carrier of acyl groups.
Vitamins as coenzymes
Stages in the extraction of energy from foodstuffs.
Regulation of metabolic pathways.
LECTURE 17
Glycolysis
Objectives: To explain how glucose is broken down to pyruvate by a series of enzyme-catalysed reactions and a (small amount) of ATP synthesised at the same time.
Key structures and reactions
Formation of Fructose 1,6-bisphosphate from glucose.
Formation of glyceraldehyde 3-phosphate.
Oxidation of glyceraldehyde 3-phosphate to 3-phosphoglycerate and substrate-level phosphorylation.
Formation of pyruvate and generation of second ATP.
Overall energy yield from glycolysis.
Regulation of glycolysis.
LECTURE 18
Mitochondria, Citric acid cycle
Objectives: To outline the role of mitochondria in the cell in the breakdown of sugars, lipids and amino-acids, and the role of the Citric Acid cycle in delivering the reducing power released into respiratory electron transfer.
Structure and function of mitochondria
Prokaryotic origin of mitochondria
Pyruvate dehydrogenase
Reactions of citric acid cycle
NAD
+
Dehydrogenases
Stoichiometry of citric acid cycle
Regulation of Pyruvate dehydrogenase and citric acid cycle
MR. J. PUDDEFOOT (LECTURES 19-22)
LECTURE 19
Mitochondria, Membrane bound electron transfer and ATP synthesis
Objectives: To explain how respiratory electron transfer generates a transmembrane electrochemical gradient of protons which drives the synthesis of (a lot of) ATP as an energy store and currency (and other energy-requiring membrane-linked processes).
Redox potential difference
Membrane-bound Respiratory Electron transfer chain, three proton pumps linked by two mobile electron carriers.
Respiratory chain complexes and redox components.
Chemiosmotic hypothesis, respiratory electron flow and ATP synthesis (oxidative phosphorylation) are linked by a transmembrane electrochemical gradient of protons.
ATP synthase.
Power transmission by proton gradients: a central motif of bioenergetics.
LECTURE 20
Chloroplasts, (Benson) Calvin cycle, photosynthetic electron transfer and photophosphorylation .
Objectives: To outline the role of chloroplasts in the biosynthesis of sugars, lipids and assimilation of sulphur and nitrogen. To show how light energy drives photosynthetic electron transfer which synthesises ATP by generating an electrochemical gradient of protons.
Structure and function of chloroplasts, and their prokaryotic origin.
Chlorophylls trap solar energy.
Antenna chlorophylls and the reaction centre.
Photosynthetic electron transfer chain.
Photophosphorylation is driven by a proton motive force.
(Benson) Calvin cycle.
RUBISCO, and subsequent reactions.
Starch and sucrose as storage products.
Energy required to fix carbon.
Thioredoxin co-ordinates light and dark reactions.
LECTURE 21
Molecular Motors, Muscle
Objectives: To introduce the proteins that make up muscle and explain how ATP hydrolysis drives muscle contraction.
Thick and thin protein filaments, myosin, actin, tropomyosin and the troponin complex.
Muscle contraction involves thick and thin filaments sliding past each other.
Myosin forms thick filaments, hydrolyses ATP and reversibly binds actin.
Structure of myosin and actin.
Dissociation of ADP from myosin induces conformational change in myosin, a change in the affinity of myosin for actin, and thus muscle contraction.
LECTURE 22
Molecular Motors, Muscle (cont), Microtubules and the flagellar motor.
Objectives: To explain how muscle contraction is regulated by calcium, mention the role of calcium in cellular regulation, and introduce other types of molecular motors.
Regulation of muscle contraction by calcium
Cytoskeleton and microtubules.
Eukaryotic cilia and flagella.
Kinesin.
Bacterial flagellar motor, driven by a proton motive force.