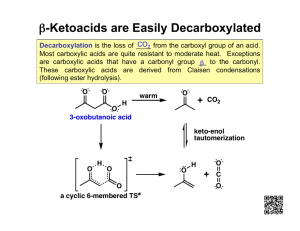
4.1.3 Carboxylic acids Booklet
advertisement

Carboxylic acids contain the carboxyl functional group. The hydroxyl group –OH, and the carbonyl group, C=O, make the carboxylic acid more polar than either an alcohol or a carbonyl. Naming of carboxylic acids Remove the final –e from the alkane and add the ending -oic acid Examples CH3CH2CH2CH2CH2CH2CH2COOH Name: octanoic acid Name: propanoic acid O C2H5 C OH Like the aldehydes, the carboxylic acid carbon is always number one in the chain although again the number one is not included in the name. H H 4 C 3 H Cl H C 2 C H H CH3 O 1 C Name: 3-chlorobutanoic acid Name: 3-methylhexanoic acid OH O OH Student activity 1 1. Name each of the following molecules O C4H9 C OH ……………………………………………………………………………… 1 CH3 H3C C O C ……………………………………………………………………………… OH H O C2H5 C ……………………………………………………………………………… OH O OH ……………………………………………………………………………… O OH ……………………………………………………………………………… 2. Draw each of the following structures, using full displayed formula 2-methylnonanoic acid methanoic acid butan-1,4-dioic acid 3-methylbutanoic acid Reactions of Carboxylic acids Carboxylic acids are like any other acid, only they are weak in comparison to acids such as They will carry out the typical reactions of acids, reacting with metals, bases and carbonates. How is the term acid defined? ……………………………………………………………………………… Why are carboxylic acids defined as weak acids? ………………………………………………………………………………………………. 2 Reactions of carboxylic acids Write fully balanced equations for the reaction between a carboxylic acid (ethanoic acid) and a:(a) Base (Sodium Hydroxide) ………………………………………………………………………………………………. The product formed in the reaction above is a salt because an acid reacts with a base to produce salt and water. In the above reaction we call the salt a carboxylate. Because the acid is ethanoic acid, the product is an ethanoate. (b) Metal (Sodium) ………………………………………………………………………………………………. (c) Carbonate (Sodium carbonate) ………………………………………………………………………………………………. Note that although carboxylic acids do react with carbonates and Mg at room temperature-phenols don’t The carboxylate ion The carboxylate ion is formed whenever a carboxylic acid is added to an alkali: Ionic Bonding H H O O H C C + OH- H OH H C H2O O- H Carboxylic acids exist in acid conditions. + C Carboxylates exist in alkali conditions. d) Esterification i) The reaction between an alcohol and a carboxylic acid. The reaction requires the use of a sulphuric acid catalyst. The reaction is reversible and reaches equilibrium. Write the equation for the reaction between ethanoic acid and methanol. …………………………………………………………………………………………………………. It is sometimes easier to draw out the structures in order to work out the product. If you draw them this way you should always get the answer correct. H H C H O + C H H O H O H ethanoic acid C CH3 H methanol 3 O C + O H2O CH3 methylethanoate water i) The reaction between an alcohol and an acid anhydride. Below is ethanoic anhydride (acetic anhydride). Write the equation for the reaction between ethanoic anhydride and methanol. It produces an ethanoic acid molecule as well as the ester. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. Student activity 2 1. What are the forces in between the molecules (vdw, pd or H-bonding)? Would you expect the acid to be soluble in water? 2. Draw a diagram to show the interaction between ethanoic acid and water. There are a number of other organic materials which can be made directly from a carboxylic acid, some of which have been discussed above. These include: Carboxylate salts Esters Acyl chlorides Amides Acid Anydrides 4 Carboxylates are ionic compounds that are bonded together by strong electrostatic forces of attraction. Properties: O C4H9 C O-Na+ Esters contain the following functional group: They are made when a carboxylic acid, in the presence of an acid catalyst, or an acid anhydride, reacts with an alcohol. Naming Esters Propanoate from propanoic acid H H3C O C Name :…………………………………. C ………………………………………….. O H C2H5 ethyl from ethanol The alcohol part comes first as the ………….yl The carboxylic acid part comes next as the …………..oate Student activity 3 1. Name each of the following esters H O Name:………………………………………………………………….. H C H C O C3H7 5 H3C H H C C H H O O H H C H Name:………………………………………………………………….. C C3H7 O Name:………………………………………………………………….. C O C2H5 2. Name the products formed between each of the following carboxylic acids and alcohols in the presence of sulphuric acid. Draw the full displayed formula for each of the products. Write a fully balanced equation for each reaction a) Ethanol and Ethanoic acid ……………………………………………………………………………………………………….. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. b) Pentan-1-ol and butanoic acid …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. c) Butan-1-ol and propanoic acid …………………………………………………………………………………………………………. ………………………………………………………………………………………………………….. ………………………………………………………………………………………………………….. Remember - an anhydride has the functional group shown. When it reacts with an alcohol R’OH, as well as the ester RCOOR’, we also make a molecule of carboxylic acid RCOOH. d) Ethanoic anhydride and propan-1-ol …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. 6 e) Propanoic anhydride and methanol …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. 1. 2. 3. 4. 7 Reactions of Esters 1. Acid Hydrolysis Hydrolysis is the reverse of esterification. Esters are unreactive with water and an acid catalyst is required to catalyse the reaction. Hydrolyis breaks down an ester to produce a carboxylic acid and an alcohol. It literally means the splitting of water by reaction with the ester. Equation for the hydrolysis of propylethanoate. ……………………………………………………………………………………………………….. 2. Base Hydrolysis (alkaline hydrolysis) http://estream.loreto.ac.uk/view.aspx?id=4755~4v~6cmREL7c The reaction with a base is called saponification (soap making). The reaction cannot be reversed. Equation: ……………………………………………………………………………………………………….. Uses of esters Esters are used as solvents in the chemical industry and as adhesives. Nail varnish remover and board pens contain ethylethanoate as a solvent. The flavours and fragrances of different esters are widely used to produce food flavours and perfumes. Ester Octyl Ethanoate flavour Orange Methyl benzoate Marzipan Methyl butanoate Pineapple Ethyl deca-2,4-dienoate Pears structure Extension Using radioactive oxygen atoms 18O and a Geiger Muller tube, describe how you could work out whether it is the C-O bond of the acid end of the ester or the O-R’ of the alcohol end of the ester which breaks during hydrolysis. This should lead you to think about the mechanism of hydrolysis which involves a nucleophilic attack on the carbonyl carbon. 8 Student activity 4 Read the passage below and underline the words which have the following meanings: a) b) c) d) e) a particle which contains covalent bonding; propane-1,2,3-triol a carbon with four different groups attached; a molecule which contains double or multiple bonds; blood vessels which take blood away from the heart. Triglycerides, saturated and unsaturated fats What are triglycerides? Triglycerides are formed from a single molecule of glycerol, combined with three fatty acids on each of the OH groups, and make up most of fats digested by humans. Ester bonds form between each fatty acid and the glycerol molecule. Below is the general structure of a triglyceride. How are trans-fatty acids linked to heart disease? Unsaturated fat is a fat molecule containing one or more double bonds between the carbon atoms. The process of hydrogenation is intended to add hydrogen atoms to cis-unsaturated fats, eliminating a double bond and making them more saturated. These saturated fats have a higher melting point, which makes them attractive for baking and extends their shelflife. However, the process frequently has a side effect that turns some cis-isomers into trans-unsaturated fats instead of hydrogenating them completely. This is another class trans fats, e.g vaccenic acid, which occurs naturally in trace amounts in meat and dairy products from ruminants. Unlike other dietary fats, trans fats are not essential, and they do not promote good health. The consumption of trans fats increases one's risk of coronary heart disease by raising levels of "bad" LDL (low-density lipoprotein) cholesterol and lowering levels of "good" HDL (high-density lipoprotein) cholesterol. It is the fact that the HDLs remove cholesterol from the blood whereas LDLs do not which leads to a build up of cholesterol in the arteries and their thickening. Health authorities worldwide recommend that consumption of trans fat be reduced to trace amounts. Trans fats from partially hydrogenated oils are more harmful than naturally occurring oils. 9 Cardiovascular disease involves the heart or blood vessels (arteries and veins). While the term technically refers to any disease that affects the cardiovascular system, it is usually used to refer to those related to atherosclerosis (arterial disease). Biodiesel refers to a vegetable oil or animal fat based diesel fuel consisting of long-chain alkyl (methyl, propyl or ethyl) esters. Biodiesel is typically made by chemically-reacting lipids (e.g., vegetable oil, animal fat (tallow)) and alcohol (or glycerol). These types of fuel are becoming increasingly important as the world’s reserves of fossil fuels are being depleted. Student activity 5 1. Show the triglyceride formed when glycerol reacts with dodecanoic acid (12 C atoms) 2. Show the triglyceride formed from the reaction of oleic acid and glycerol. |Oleic Acid 3. Label the following as cis or trans. 10 4. How might the melting points of the cis and trans (Z and E) isomers of a fatty acid differ? By looking at their shapes, you should be able to see that trans isomers will be able to pack closer together so their will be more Vd W forces so the melting points will be higher. Jun12 11