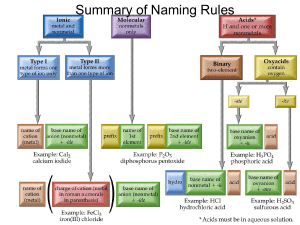
NAMING IONIC COMPOUNDS (p 156)
advertisement

IONIC COMPOUNDS II (p 124 Again) Today, we'll work with ionic compounds made from Transitional Metals. Since transitional metals have more than one possible oxidation number, you put the oxidation number in the name of the compound! Example: If you have a compound with Cobalt and Oxygen, and the oxidation number for Cobalt is +2, then the name is Cobalt (II) Oxide and its formula is CoO. (Oxygen is -2). If the Cobalt has an oxidation number of +3, then it's called Cobalt (III) Oxide, and the formula is Co2O3! This time, I will give you the name of the compound, and you'll have to figure out its formula! 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) Iron (II) Oxide (Hint: Iron's symbol is Fe!) Iron (III) Oxide Chromium (II) Fluoride Chromium (III) Bromide Chromium (VI) Chloride Chromium (VI) Sulfide Vanadium (V) Nitride Manganese (VII) Selenide Copper (I) Phosphide Silver (II) Iodide ___________________ ___________________ ___________________ ___________________ ___________________ ___________________ ___________________ ___________________ ___________________ ___________________