hybrid orbitals are formed by a mixing of the
advertisement

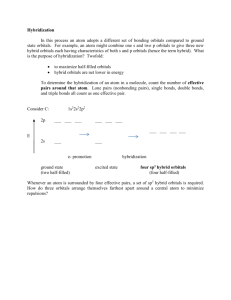
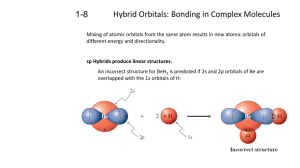
Hybridization Captured speech text from: http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/056_Hybridization.MOV Hybrid orbitals are formed by a mixing of the hydrogen-like s, p and d orbitals, that characterize a free atom. The three p orbitals are directed along the “x”, “y” and “z” axes. When we mix an s and a px orbital we produce two hybrid orbitals, called sp, pointed in opposite directions along the x axis. Mixing an s with a px and a py orbitals, produces three equivalent hybrid orbitals in the x-y plain. We call these sp² hybrid orbitals. Notice at the hybrid orbitals has major lobes which contain most of the electron density in the orbital. These are used in bonding at outer atoms. Mixing of the s with all three p orbitals, resolves in formation of four equivalent sp³ hybrid orbitals, directed on the apices of a tetrahedron. Let’s apply the concept of hybridization to ammonia. Here is the Lewis structure for ammonia. There are four electron pairs in the nitrogen valence orbitals. We note an ammonia molecule has a pyramidal shape. The shape can be explained by assuming that the central nitrogen atom employs sp³ hybrid orbitals. The unshared electron pair occupies the fourth sp³ hybrid orbital.