Supplemental Notes
advertisement

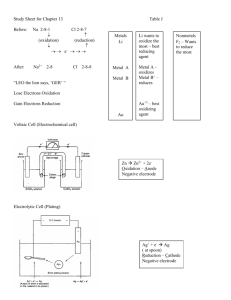
Chapter 16 Electrochemistry Mr. von Werder Supplemental Notes Review some ideas that help us understand electrochemical cells: oxidation and reduction is the result of electron transfer electrical energy is a flow of electrons (current) metals are good conductors (because of the loosely held valence electrons) electrolytes can also conduct a current (because of mobile ions) Electrochemical cell: redox chemical system that either produces or uses electrical energy uses a spontaneous flow of electrons to our advantage voltaic cells use spontaneous redox reactions to generate electricity water downhill analogy electrodes: - anode, oxidation occurs + cathode, reduction occurs example: batteries of various types fuel cells electrolytic cells use electricity (from external source) to drive a nonspontaneous redox reaction water uphill analogy electrodes: + anode, oxidation occurs - cathode, reduction occurs examples: electrolysis electroplating Electrochemical Cells Voltaic Cell Setup Intro: Metals can replace metal ions in solution that are below them on the activity series. This happens by electron transfer - more active metal to the less active metal. This transfer will happen directly if the metal is put directly in the solution Example: Zn(s) + CuSO4(aq) ---> ZnSO4(aq) + Cu(s) However, if the oxidation and reduction processes are separated the flow of electrons can be made to go through an external wire - then useful work can be made of the electron flow (electricity) Voltaic cell - spontaneous redox reaction that produces electricity - harness the electricity by rerouting electrons through an external wire, to light a bulb, play a radio, etc. - this is the basis for what we commonly call “batteries” Components: OXIDATION HALF-CELL ANODE - the electrode where oxidation occurs - has a negative sign (-) in a voltaic cell (here the electrons are released and move through a wire to the cathode) - often is the metal that will be oxidized (part of the reaction) - sometimes is just a necessary conductor (graphite or platinum electrode), but is not part of the reaction SOLUTION - contains the cations of the element being oxidized (necessary?) - also anions are present for neutrality REDUCTION HALF-CELL CATHODE + electrode where reduction occurs + has a positive sign (+) in a voltaic cell (here the electrons are accepted through a wire from the anode) + often is the metal that will be reduced (part of the reaction) + sometimes is just a necessary conductor (graphite or platinum electrode), but is not part of the reaction SOLUTION - contains the cations of the element being reduced - also anions are present for neutrality SALT BRIDGE - connects the two half-cells - contains an electrolyte solution (ions that will not interfere) - allows for ions to flow between cells - (completes the circuit; maintains neutrality in each half-cell) - porous barrier between half-cells can serve the same purpose (porous disk(p.684), or a porous cup (demo)) WIRE - to connect the anode to the cathode - allows for the transfer of electrons to occur indirectly Cell Potential Electrons will spontaneously flow from higher to lower potential energy position. The difference in potential energy is released as electrical energy as the electrons move and can be made to do work (light a bulb, play a radio, run a motor). Cell potential - difference in electrical potential energy between the two electrodes - measured in volts (named after who?) (sometimes called cell voltage) - measured with a voltmeter (or DC setting on a multimeter) - DEPENDS on ● substances reacting ● temperature ● concentrations ● pressures of gases (if involved) Standard Cell Potential In order to compare different cells, standard conditions have been established. ● temperature of 25°C ● concentrations of 1 M ● pressures of 1 atm Standard Electrode Potentials Standard cell potential (Ecell) is the sum of the potentials of the two half-cells Eox is the oxidation potential Ecell = Eox + Ered Ered is the reduction potential Eox and Ered cannot be measured directly, so they are measured against a standard and then tabulated for reference Standard Hydrogen Electrode (SHE) is the reference reduction of H+(aq) to H2(g) is assigned a potential of 0.0 V Table of Standard Reduction Potentials – Supplemental HANDOUT - more positive the Ered (from table), the more likely to be reduced - more negative the Ered (from table), the more likely to be oxidized - calculating an overall cell potential (Ecell) look up the two half-reactions involved on the table flip the one that is more negative and its E to represent oxidation Common Batteries The Common Dry Cell “Dry” because the electrolyte is in the form of a paste (rather than aqueous solution) See Figure 21-13, page 698. Anode: Zinc casing: zinc is oxidized Zn ---> Zn2+ + 2eCathode: Graphite electrode; paste consisting of MnO2, and other things Mn (from MnO2) is reduced Pros: low cost Cons: not rechargeable, high current draw discharges cell quickly, poor shelf life The Alkaline Dry Cell “Alkaline” because the electrolyte paste contains KOH (a base – alkaline) See Figure 21-13, page 698. Anode: Paste consisting of powdered Zn, KOH, water: zinc is oxidized Cathode: Paste consisting of MnO2, and other things: Mn (from MnO2) is reduced Pros: longer shelf life, maintains a steady voltage, more energy than dry cell Cons: more expensive than dry cell, also not rechargeable Lead Storage Battery commonly found in your car Sulfuric acid is the electrolyte, so it is known as “battery acid” Anode: Pb plates; Pb is oxidized (0 to +2) Cathode: PbO2 plates; Pb is reduced (+4 to +2) Pb + SO42- --> PbSO4 + 2ePbO2 + 4H+ + SO42- + 2e- --> PbSO4 + 2H2O Pb + PbO2 + 4H+ + 2SO42- --> PbSO4 + 2H2O Eox = +0.35 Ered = +1.69 Ecell = +2.04 How do you get a 12-V car battery? Arrange six of these cells together --- a “battery” Alternator in your car reverses the electron flow in the battery and recharges it. Pros: rechargeable, relatively cheap Cons: massive, disposal concerns will all of the lead (a heavy metal contaminant) Nickel-Cadmium battery Rechargeable batteries (nicad for nickel-cadmium) used in many ‘cordless’ devices. Anode: Cd metal is oxidized Cathode: Nickel (from NiO2) is reduced Pros: lightweight, constant voltage, rechargeable Cons: ‘discharge memory’, disposal concerns (cadmium is toxic) Fuel Cells Most common fuel cell is the hydrogen/oxygen fuel cell Hydrogen and oxygen must be continually supplied to the cell Anode: Hydrogen is oxidized 2 H2 + 4 OH- --> 4 H2O + 4eCathode: Oxygen is reduced O2 + 2 H2O + 4e- --> 4 OH- Eox = +0.83 Ered = +0.40 2 H2 + O2 --> 2 H2O Ecell = +1.23 Biggest selling point is the efficiency – a reaction that may be combusted to produce heat to heat water to spin a turbine to generate electricity … can directly create electricity. Pros: don’t run down (as long as fuel is provided), very efficient, drinking water Cons: expensive, not very portable Electrolytic Cells Electrolytic cells use electricity to cause a nonspontaneous redox reaction to occur Components, See source of direct current (for example, a battery - voltaic cell) electrodes anode; where oxidation occurs, but is in the electrolytic cell cathode; where reduction occurs, but is - often the electrodes are inert, and are not part of the reaction - electrons are driven from anode to cathode by the battery (an electron pump) solution (or liquid) containing the reactants Electrolysis of Molten Sodium Chloride First, NaCl needs to be melted, so it be heated to 801C (Why so high a m.p.?) Anode: chlorine is oxidized 2 Cl-(l) --> Cl2(g) + 2eEox = -1.36 + Cathode: sodium is reduced 2 Na (l) + 2e --> 2 Na(l) Ered = -2.71 2 NaCl(l) --> 2 Na(l) + Cl2(g) Ecell = -4.07 Notice the negative Ecell -- a nonspontaneous reaction, requiring electricity to occur Figure 21-18, page 706, Down’s cell Practical way of getting metallic sodium Electrolysis of Water First, an electrolyte needs to be added to the water -> to provide more ions -> to allow more current to flow. Anode: O2 is formed 2 H2O --> 4 H+ + O2 + 4eCathode: H2 is formed 4 H2O + 4e- --> 2 H2 + 4 OH2 H2O --> 2 H2 + O2 NOT a practical way to obtain quantities of oxygen (easier to liquefy and distill air) - but, the reduction to form hydrogen in aqueous solutions is commonly used Electrolysis of Aqeous Sodium Chloride Anode: Cathode: Cl2 is formed H2 is formed 2 Cl- --> Cl2 + 2e2 H2O + 2e- --> H2 + 2 OH2 Cl- + 2 H2O --> Cl2 + H2 + 2 OHCommercially this is an important reaction: it produces three valuable products: Chlorine gas, hydrogen gas, and sodium hydroxide solution Electroplating Electroplating: uses electrolysis to deposit a thin coating of metal on an object Uses: - to protect another metal (chrome plating, tin cans) - decorative (silver and gold plating) Anode: plating metal, to be oxidized Cu --> Cu2+ + 2eCathode: object to be plated, reduction occurs here Cu2+ + 2e- --> Cu(s)