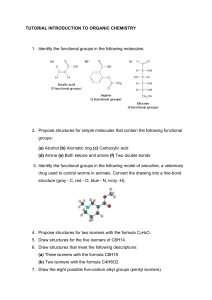
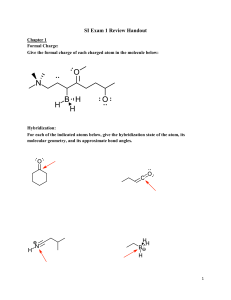
1. What functional groups are present in these molecules? What is the DU of each? A. B. D. E. F. G. C. H. 2. Draw all the isomers for each of these formulas. A. C3H8O B. C4H9Cl C. C4H8 D. C4H8O 3. Determine whether these structures represent the same compound or isomers: A. C. B. D. 4. Draw all the important resonance structures for these species A. C. B. D. 5. Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbitals that are overlapping to form it A. C. B. D. 6. Show the conjugate acid of each of these species: A. C. B. D. 7. Explain which compound is the stronger acid: A. C. B. D. 8. Explain which species is the stronger base: A. C. B. D. Assigned question for each group are: 1. 2. 3. 4. 5. 6. 7. 8. Group 1 – Q1.A, Q2.D, Q3.B, Q4.A, Q5.D, Q6.D, Q7.A, Q8.C Group 2 – Q1.B, Q2.C, Q3.C, Q4.C, Q5.C, Q6.C, Q7.B, Q8.A Group 3 – Q1.C, Q2.B, Q3.A, Q4.B, Q5.B, Q6.A, Q7.C, Q8.D Group 4 – Q1.D, Q2.A, Q3.D, Q4.D, Q5.A, Q6.B, Q7.D, Q8.B Group 5 – Q1.E, Q2.B, Q3.D, Q4.A, Q5.D, Q6.D, Q7.A, Q8.D Group 6 – Q1.F, Q2.C, Q3.A, Q4.C, Q5.B, Q6.A, Q7.C, Q8.C Group 7 – Q1.G, Q2.D, Q3.C, Q4.B, Q5.A, Q6.C, Q7.B, Q8.B Group 8 – Q1.H, Q2.A, Q3.A, Q4.D, Q5.C, Q6.B, Q7.D, Q8.A