Identifying R and S Configurations Using Fischer Projections:
advertisement

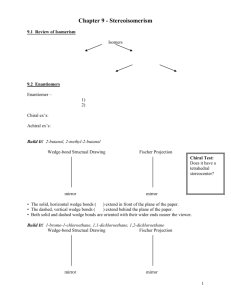
Identifying R and S Configurations Using Fischer Projections: Fischer projections can be reoriented (redrawn). It is important to distinguish between rotating atoms around single bonds (changes only the conformation) and changing the configuration (breaking bonds and reforming them in different positions). Rules for redrawing Fischer projections: 1. Exchange any 2 groups around the same chiral center converts a Fischer projection into its enantiomer. a b exchange a) & b) b d d a c c original molecule mirror image (enantiomer) 2. Exchanging any 2 pairs of groups (exchanging any 2 groups twice) gives the original configuration. 3. Exchanging any 3 groups in sequence around a chiral center (while holding 1 group in place) gives the same molecule. This is equivalent to rule 2, i.e., exchanging any 2 groups twice. H F H Br Br Cl original molecule Hold Cl in place and exchange the other 3 groups in sequence. F Cl same molecule Rules for rotations of Fischer projections: 1. Rotation of the entire projection by 180 gives the same molecule 2. Rotation of the entire projection by 90 or 270 gives the mirror image (enantiomer). Rules for determining R or S configuration of Fischer projections: 1. Draw the molecule in a Fischer projection 2. Use any of the previously stated rules for redrawing or rotating Fischer projections, which retain configuration and place the group with lowest priority at the top or bottom of the Fischer projection. 3. Draw a curved arrow on the page pointing from 1 2 3 (ignore 4). 4. A clockwise arrow indicates R configuration. A counterclockwise arrow indicates S configuration. clockwise = R counterclockwise = S Enantiomers A chiral C is hybridized (has 4 bonds) and is bonded to 4 different groups. A molecule containing a chiral C exists in 2 geometrically different forms, i.e., a pair of enantiomers (geometrically opposite isomers). A pair of enantiomers are non superimposable mirror images. sp3 mirror Br H H Br H3C H trans-1-bromo-2-methylcyclopentane A pair of enantiomers, i.e., non superimposable mirror images. H CH3 Pairs of enantiomers have identical physical and chemical properties (bp, mp, solubility, etc.) but differ only in their effect on plane polarized light. A chiral molecule rotates the plane of polarized light (is optically active). The opposite enantiomer rotates the plane of polarized light by the same amount but in the opposite direction. A racemic mixture (50:50 mixture) of a pair of enantiomers is optically inactive. The effect of one enantiomer cancels the optical rotation produced by its opposite enantiomer. Meso Compounds A meso compound has 2 or more chiral centres but has a plane of symmetry (a plane which divides a molecule into 2 halves that are mirror images-not necessarily superimposable). Whenever a plane of symmetry is present, it is found that the 2 opposite enantiomers are actually one and the same compound, i.e., their mirror images are superimposable. A compound containing chiral centres and a plane of symmetry is called a meso compound. Meso compounds are optically inactive. Each half of the molecule rotates the plane of polarized light in opposite directions and the optical effects cancel. Both meso compounds and racemic mixtures are optically inactive. Consider 1,2,3,4,5, 6-hexanehexol: This alditol has 4 chiral C's and thus can exist in up to a maximum of 24 or 16 stereoisomeric forms or 24-1 or 8 pairs enantiomers However, more than 1 of the 8 possible pairs of enantiomers exist as meso forms. D-galactitol is a meso compound (as seen by its plane of symmetry). Hence, D-galactitol and its mirror image, L-galactitol are one and the same compound. mirror plane of symmetry CH2OH H HO HO H OH H H OH CH2OH D-galactitol CH2OH HO H H H HO OH OH H CH2OH CH2OH CH2OH CH2OH CH2OH L-galactitol Assign R and S configurations to each chiral C in D-galactitol and name this compound using IUPAC nomenclature. Draw and name any other meso forms of 1,2,3,4,5, 6-hexanehexol.