combustion of ethyne
advertisement

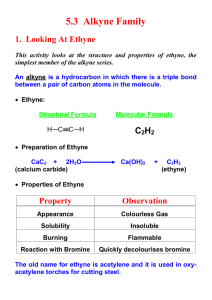
Lab – Effect of Oxygen on Combustion of Ethyne Purpose: To determine the effect of differing amounts of oxygen on the combustion of ethyne ( acetylene). Hypothesis: The tube with _____________% of ethyne will show complete combustion. Other tubes with less oxygen will show ____________________________ combustion. (2) Prelab: a) Write out and balance the equation for the reaction of ethyne ( C2H2)with oxygen. (2) b) Write out and balance the reaction of calcium carbide with water. (2) Materials: list all materials used – be specific and draw a fully labeled diagram of the experimental set-up. (6) Procedure: 1) 2) 3) 4) 5) 6) 7) 8) Put on goggles, tie back hair, push in stools and clear aisles before beginning. Obtain three tubes of ethyne gas- a) 100% ethyne, b) 50% ethyne and c) 10% ethyne. Clamp a test tube clamp to a retort stand. Clamp test tube A to the retort stand making sure it is secure and is pointing away from yourself. Place a blast shield between your group and the lab materials. Reach around the blast shield and remove the stopper from the tube and quickly bring a burning splint to the mouth of the tube. Observe the combustion. Clean tube after it cools and wash and dry it. Repeat steps 4-6 with test tube B then test tube C. Clean up all materials. Observations: Describe each reaction and the visible products produced. (put observations into short paragraphs. (6) Discussion Questions : 1) 2) 3) 4) 5) What are the dangers of incomplete combustion? ( give at least two and explain each) (4) What are some uses of ethyne? ( give two minimum) (2) How does CO2 from combustion affect the atmosphere and ocean pH? (4) How would a clogged air filter affect the operation of a car? (3) Explain why the balanced equation for the combustion of ethyne will not give you the correct proportions of air and ethyne needed for complete combustion. (2)