File - Garbally Chemistry
advertisement

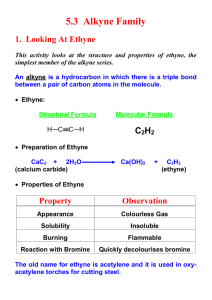
Chapter 21 Fuel and Heats of Reaction. Organic chemistry is defined as the study of the compounds of carbon. A hydrocarbon is a compounds of carbon and hydrogen only. Types of hydrocarbon. Aliphatic- have molecules in chains or rings of carbon atoms. Aromatic - hydrocarbons with a Benzene ring of 6 carbon atoms, where the bonds are intermediate between single and double bonds. Aliphatic hydrocarbons. A homologous series is a group of compounds with similar chemical properties due to having the same functional group. They have a gradual variation in physical properties and they differ from each other by CH2. A functional group is a group of atoms within the molecule which, due to its structure, gives the molecule its characteristics.(-OH functional group gives alcohols their characteristics) Structural Isomers Structural Isomers are compounds with the same molecular formula but different structural formulas. The 3 main classes of Hydrocarbons are Alkanes, Alkenes and Alkynes. The Alkanes Formerly known as the ‘paraffins’ (from the Latin meaning ‘lacking affinity’) the alkanes are a group of hydrocarbons which conform to the general formula CnH2n+2, where n is greater than or equal to 1. They are saturated compounds. They consist of carbon atoms joined by four single covalent bonds to either hydrogen or other carbon atoms. The first member of the group is methane (CH4), the second is ethane (C2H6), and the third is propane (C3H8), as illustrated in Fig. 3.2. By drawing their structural formulae we can see that each differs from the next by replacing a hydrogen with a CH3 group, i.e. an extra CH2 is inserted in the chain. Physical properties C1-C4 –Gases C5-C15-Liquids C15----- Waxy solids Alkanes are non polar, They are insoluble in water but soluble in non polar solvents like cyclohexane and methylbenzene Alkenes The alkenes are the second homologous series .They are unsaturated. They have the general formula CnH2n. Each alkene contains a carbon-carbon double bond. Alkenes are said to be unsaturated. The first two are: Ethene C2H4 Propene C3H6 For example, with C4H8, it isn't too difficult to come up with these three structural isomers: Physical properties C2-C4 Gases at room temp C5--- Liquids and gases Alkenes are non polar, They are insoluble in water but soluble in non polar solvents like cyclohexane and methylbenzene. Alkynes Alkynes are a group of hydrocarbons which conform to the general formula CnH2n-2. Each alkyne contains a C-C triple bond. They are unsaturated Physical properties Alkynes are non polar, The lower members are gases at room tempwhile higher alkynes arte liquid or solid They are insoluble in water but soluble in non polar solvents like cyclohexane and methylbenzene. Ethyne Ethyne or Acetylene, colourless, odourless, flammable gas, HCCH, slightly lighter than air. As ordinarily prepared it has an unpleasant odour due to impurities. it is usually made commercially by the reaction of calcium carbide with water. Although ethyne can be liquefied at ordinary temperatures with high pressure, it is violently explosive as a liquid. Ethyne burns in air with a hot and brilliant flame. It was formerly much used as an illuminant and is now mainly used in the oxyacetylene torch, in which ethyne is burned in oxygen, producing a very hot flame used for welding and cutting metal. Ethyne is also used in chemical synthesis, particularly in the manufacture of chloroethene (vinyl chloride) for plastics, ethanal (acetaldehyde), and the neoprene type of synthetic rubber. Ethyne has a melting point of -81° C (-113.8° F) and a boiling point of -57° C (70.6° F). Preparation of Ethene Ethanol is dehydrated using hot aluminium oxide as a catalyst to produce ethene. Al2O3 C2H5OH → C2H4 + H2O heat The ethene gas formed in the reaction is insoluble in water, and is therefore collected by downward displacement of water. Investigation of Properties 1. Ignite the gas in one of the test tubes. Describe the flame (coloured or clear, smoky or clean). Pour a few drops of limewater into the test tube. Stopper, shake well and record what you see. 2. Add a few drops of diluted bromine water to the second test tube of gas. Stopper and shake well. Record what you see. 3. Add a few drops of acidified potassium manganate(VII) solution to a third test tube of the gas. Stopper immediately and shake well. Record what you see. Preparation and properties of ethyne Student Material Theory Calcium dicarbide reacts with water producing ethyne and calcium hydroxide: CaC2 + 2H2O → C2H2 + Ca(OH)2 The gas usually contains hydrogen sulfide and phosphine as impurities, caused by the hydrolysis of traces of calcium sulfide and calcium phosphide present in the calcium dicarbide. The impurities, which cause an unpleasant odour, can be removed by bubbling the gas through acidified copper sulfate solution. (2) Investigation of Properties 1. Ignite the gas in one of the test tubes. Describe the flame. Add a few drops of limewater to the test-tube and shake well. Describe what happens. 2. Add a few drops of a solution of bromine water to a test tube of gas, stopper quickly and shake well. Describe what you see. 3. Add a few drops of acidified potassium manganate solution to a test tube of gas, stopper quickly and shake well. Describe what you see.







![LC Fuels and Thermochemistry [PDF Document]](http://s3.studylib.net/store/data/008241147_1-6ebc9d449a7896c353ddca434fe5df53-300x300.png)
