File
advertisement

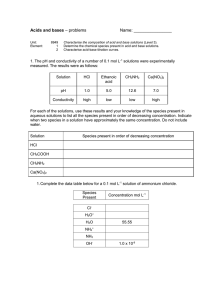
CHEMISTRY 3.7 WORKSHEET SEVEN 1 (a) (b) Name: AQUEOUS SYSTEMS Titrations 0.1 mol L-1 NaOH solution is added to 8 mL of 0.1 mol L-1 HCl in a titration experiment. (i) What is the pH of the original acid solution. (ii) What is the pH of the final solution when 16 mL of NaOH solution has been added. (iii) What is the pH of the solution at equivalence point? (iv) Sketch the titration curve on the following graph axes. Using a different pen colour, sketch the graph curve if the experiment was repeated using 0.1 mol L-1 ethanoic acid instead of hydrochloric acid and circle the buffer zone. (Hint: Calculate the new initial pH and buffer pH) (Given Ka (CH3COOH) = 1.74 x 10-5 ) (c) Indicators are themselves weak acid/conjugate base systems and have their own pKa values. Suggest a suitable pKa for an indicator to use in the (i) 2. (a) (b) HCl titration. (ii) CH3COOH titration 0.1 mol L-1 HCl is added to 8 mL 0.1 mol L-1 NH3 solution in a titration experiment. Ka (NH4+) = 5.70 x 10-10 (i) What is the initial pH of the ammonia solution? (ii) What is the pH after 4 mL of HCl has been added? (iii) What is the pKa of a suitable indicator for this titration? (iv) Sketch the titration curve on the following graph axes. (i) Circle the buffer zone. (ii) Calculate the final pH of the mixture after 16mL of acid has been added.