Titration: Definition, Calculations, and Practice Problems
advertisement

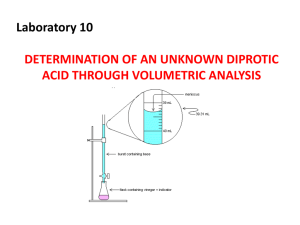
Titration Titration helps you determine the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. It is when you add one solution of a known concentration to a known volume of another solution of unknown concentration until the reaction reaches neutralization. This will be indicated by a color change. for example (to find the concentration of an acid solution, you would need to titrate the acid solution with a solution of a base of known concentration.) Many indicators used for titrations are weak acids. The point at which the indicator used in a titration changes color is called the end point of the titration. The end points can be basic, neutral or acidic. If it is basic, then you will know that the reaction has a strong base and weak acid in it. If it was neutral then you will know the reaction had a strong acid and a strong base. If it was acidic, then you would know that the reaction has a strong acid and a weak base in it. Definitions Analyte: the solution of unknown concentration but known volume. End point- The point at which an indicator changes color. Equivalence point: The point at which moles of H+ ion from the acid equals moles of OH– ion from the base. example [H+] = [OH-] Indicator: is the substance which changes color when an excess of amount of titrant has been added (phenolphthalein). Neutralization reaction -a reaction in which an acid and a base in an aqueous solution react to produce a salt and water. Salt - an ionic compound made up of a cation from a base and an anion from an acid. Standard Solution or titrant– An acid/ base solution whose concentration is known Titration - determine the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. HOW TO USE TITRATION CALCULATIONS? 1. First you will need to find the number of moles of titrant that is added to reach of the endpoint. 2. Then you will have to determine the moles of analyte that must have been used. Therefore you can use stoichiometric coefficients to help you out. 3. Determine the amount of concentration of analyte that must have been in the flask. 4. Lastly, Calculate the concentration of analyte in the original sample. Practice Questions A 43.0 mL of sodium hydroxide was titrated against 32.0 mL of 0.100 M hydrochloric acid. What is the molarity of sodium hydroxide solution? Step 1: Find the moles of hydrochloric acid: Mol = M x L Mol = 0.100 mol x .032 L L HCl = .0032 mol Step 2: Write a balanced equation, and find the molar ratio between the acid and the base. HCl + NaOH NaCl + H2O Ratio:- HCl : NaOH 1 mol : 1mol NaoH = 0.0032 Mol Step 3: Find the concentration of sodium hydroxide. M (NaOH) = mol/ L M = 0.0032 mol 0.043 L NaOH = 0.0744 M TRY THIS ONE ON YOUR OWN! 14.84 mL of an HCl solution of unknown concentration is titrated with standard NaOH solution. At the equivalence point, 25.0 mL of the 0.675 M NaOH has been added. Calculate the concentration of the HCl solution. Answer- (1.14 M) If you want more practice questions, you can go on this website. http://pattersonscience.weebly.com/uploads/5/1/5/0/5150508/unit_6_lesson_10_answers_to_homew ork_on_titrations.pdf If you need more help on it, you can go on this youtube link. http://www.youtube.com/watch?v=SaM0BHboSIU